Introduction
Infections with intestinal nematodes are widespread in humans living in tropical and subtropical countries, where as a consequence of a poor sanitary infrastructure, environmental contamination with feces is high. In 2010, the estimated global burden of intestinal helminthiases was 5.2 million (Mio) disability adjusted life years (DALYs) lost.1 Infections are commonly caused by soil-transmitted helminths, namely Ascaris lumbricoides, hookworm (Ancylostoma duodenale and Necator americanus), and Trichuris trichiura. Hookworm disease accounts for the biggest part of the burden estimates (3.2 Mio DALYs), mainly because hookworms cause and contribute to iron deficiency anemia, which can negatively impact on the health of children and women of childbearing age as well as fetuses and newborn babies.2,3 Strongyloides stercoralis, an often neglected additional soil-transmitted helminth species, is infecting an estimated 30–100 Mio people,3 but no DALY burden estimates exist. Recent prevalence estimates suggest that strongyloidiasis affects between 10% and 40% of the population in many tropical and subtropical countries, but particularly in sub-Saharan Africa and Southeast Asia, infection with S. stercoralis is highly underreported.4 Strongyloidiasis can be asymptomatic or lead to cutaneous, gastrointestinal, or pulmonary symptoms, like skin rashes, abdominal pain, and abnormal wheezing, respectively.5–8 Importantly, hyperinfections evolving in immunocompromised individuals can be potentially fatal.9–12 The difficulties in correctly diagnosing this parasite are mainly responsible for its constant neglect in epidemiological mapping and burden estimations.
Recently, the will to control neglected tropical diseases has been boosted by the ambitious goal of the World Health Organization (WHO) to eliminate neglected tropical diseases or reduce their impact to levels at which they are no longer considered public health problems by 2020.13 The target for soil-transmitted helminths is to regularly treat 75% of pre-school- and school-aged children in need of treatment and achieve 75% treatment coverage in all endemic countries. In support of this goal, a considerable number of public and private partners officially committed in the “London Declaration on Neglected Tropical Diseases” from January of 2012 to help with the control of soil-transmitted helminthiases by supplying drugs and other interventions.14
Scaling up interventions to control soil-transmitted helminthiases will require a solid and timely assessment of the epidemiological situation on the basis of sensitive and specific diagnostic methods to (1) guide the initiation of interventions, (2) monitor and evaluate the impact of interventions, (3) detect anthelminthic resistance at an early stage of development in the field, (4) confirm the interruption of transmission, and (5) spot the recrudescence of infections and disease by surveillance.13,15,16 Currently applied diagnostic methods have, however, a number of drawbacks and technical limitations. The Kato–Katz thick smear method, which is the most widely used technique to assess soil-transmitted helminth prevalence and infection intensities in epidemiological surveys and helminth control programs, is a cheap and simple method but lacks sensitivity for the detection of low-intensity soil-transmitted helminth infections.17,18 The recently developed FLOTAC technique, which is also based on microscopic detection of helminth eggs in stool samples, has a higher sensitivity to identify light soil-transmitted helminth infections, and the application is gaining popularity in research studies conducted across the world.19–25 The disadvantages of the FLOTAC technique are that it requires more sophisticated laboratory equipment, such as a centrifuge and special chemicals, and is a relatively low-throughput method. For the diagnosis of S. stercoralis larvae in stool samples, the Kato–Katz method and FLOTAC are not suitable. For this purpose, the Baermann funnel26 and stool culture techniques, such as the Koga agar plate,27 stool-charcoal or -vermiculate mixtures in Petri dishes,28,29 or if possible, a combination thereof, are recommended.10,18,30 Different polymerase chain reaction (PCR) -based approaches for the detection of soil-transmitted helminth DNA or ribosomal RNA in stool samples have been developed and are increasingly promoted for monitoring and surveillance of control programs.31–35 It remains to be elucidated, however, if the sensitivity of PCR-based diagnosis of helminth infections in stool samples is considerably better than the sensitivity of direct parasitological methods, particularly if infection intensities are low.
Here, we compare and discuss multiple aspects of the diagnostic performance of the Kato–Katz method and FLOTAC, the Kato–Katz method and PCR, the Baermann method and PCR, and for the first time, FLOTAC and PCR for the diagnosis of hookworm and S. stercoralis infections. Three different statistical approaches were used to render our results comparable with a broad set of previous and future studies. Stool samples were obtained from individuals living in the Bagamoyo District in the coastal region of the United Republic of Tanzania who participated in a screening for helminth infections for the IDEA project between June of 2011 and November of 2012. The IDEA project is an African–European research initiative that aims to dissect the immunological interplay between poverty-related diseases and helminth infections (http://ec.europa.eu/research/health/infectious-diseases/neglected-diseases/projects/014_en.html).
Materials and Methods
Ethics statement.
The institutional research commissions of the Swiss Tropical and Public Health Institute (Swiss TPH; Basel, Switzerland) and the Ifakara Health Institute (IHI; Dar es Salaam, United Republic of Tanzania) approved the protocol of the IDEA project conducted at the Bagamoyo Research and Training Center (BRTC) of the IHI in the United Republic of Tanzania. The Ethikkomission beider Basel (EKBB; Basel, Switzerland; reference number 257/08) and the National Institute for Medical Research (NIMR; Dar es Salaam, United Republic of Tanzania; reference number NIMR/HQ/R.8a/Vol.IX/1098) granted ethical approval for the study.
The purpose and procedures of the study were detailed to the local district, community, and health authorities and explained to individuals eligible for screening and potential participation in one of three study arms of the IDEA project. In brief, these study arms are investigating the immunological interplay between helminth infections and malaria (arm 1), tuberculosis (arm 2), and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS; arm 3). Participants were informed that their participation was voluntary and that they could withdraw from the study at any time without additional obligation before they were invited to sign a written informed consent sheet. From all participating adult individuals and the parents or legal guardians of participating minors (children below the age of 10 years), written informed consent was obtained. In the cases that participants or their parents or guardians were illiterate, they signed by thumbprint.
Participants infected with soil-transmitted helminths were administered albendazole (400 mg single oral dose) against A. lumbricoides, hookworm, or T. trichiura, ivermectin (200 μg/kg single oral dose) against S. stercoralis, or praziquantel (40 mg/kg) against schistosome infections according to the national treatment guidelines of the United Republic of Tanzania.
Study area.
The participants whose data were included in the present analysis were children and adults residing in rural villages within the Bagamoyo District, which is located north of Dar es Salaam in the coastal region of the United Republic of Tanzania. Samples were collected between June of 2011 and November of 2012. The fresh stool specimen were examined in the Helminth Unit Laboratory of the BRTC, and preserved stool samples were analyzed with PCR in the laboratory of the NIMR-Mbeya Medical Research Center (NIMR-MMRC) in Mbeya, United Republic of Tanzania.
Field procedures.
Potential candidates for the inclusion in one of the study arms of the IDEA project were (1) children aged 6 months to 9 years living in the west catchment areas of one of six health facilities in the Bagamoyo District, (2) children aged 6 months to 9 years who presented at one of six health facilities with either asymptomatic or uncomplicated malaria, (3) children who presented at the Bagamoyo District Hospital with severe malaria, and (4) people of all age groups who were part of a community health screening conducted in remote villages in the Bagamoyo District to recruit new participants for any arm of the IDEA study. All candidates were screened for helminth infections as detailed below.
After written informed consent or thumbprint was obtained from the participant or in case of minors, the parent/legal guardian, the participant was registered, assigned a personal unique identification number, and provided with a plastic container (100 mL) for collection of a fresh morning stool sample that was to be submitted the next day before 12:00 PM to the consulted health facility or in case of the village health survey, a pre-defined meeting point in the village center. The samples were collected every day around 12:00 PM from the health facilities or the central village points in the Bagamoyo area by a fieldworker and transported by motorbike to the Helminth Unit of the BRTC.
Laboratory procedures.
All stool samples were examined in the Helminth Unit of the BRTC right after arrival by experienced laboratory technicians. The Baermann method was applied for the detection of S. stercoralis larvae.36 In brief, a walnut-sized stool sample was placed on double-layered gauze in a tea sieve within a glass funnel that was filled with tap water and exposed to electric light from below. Phototactic S. stercoralis larvae were collected after 2 hours of light exposure and visualized on microscope slides, and their number was recorded in the case report form (CRF) of the respective participant. Duplicate Kato–Katz thick smear slides were prepared from each stool sample for the detection of soil-transmitted helminth and S. mansoni eggs.37 For this purpose, filtered stool samples were filled in a 41.7 mg template, and the stool smears were incubated for ∼20 minutes before the slides were read under the microscope. The number of helminth eggs was counted and recorded species specifically. Moreover, the FLOTAC dual technique was performed for the diagnosis of soil-transmitted helminth and S. mansoni infections.38 A small subsample of each individual's stool (∼1 g) was weighed and preserved in sodium acetate-acetic acid-formalin (SAF) for examination by FLOTAC the next day, and 0.5 g stool were placed in cryotubes and frozen at −80°C for DNA extraction and examination with PCR at a later point in time. The FLOTAC dual technique was performed the next morning before new samples arrived. We used flotation solution 2 (FS2; saturated sodium chloride [NaCl] solution; specific gravity [s.g.] = 1.20) and FS7 (zinc sulfate [ZnSO4 · 7H2O] solution; s.g. = 1.35).
For the DNA isolation, we followed the procedure described by Verweij and others.39 All DNA samples were stored at −20°C and transferred on ice to the NIMR-MMRC, where PCR amplification and detection were conducted in June and November of 2012.
A multiplex real-time PCR was used for the simultaneous detection of A. lumbricoides, N. americanus, S. mansoni, and S. stercoralis DNA in fecal samples.31,32,40,41 For DNA amplification, 5 μL DNA extracted from 0.1 g stool specimens were used as a template in a final volume of 25 μL with PCR buffer (HotstarTaq Master Mix [5 mM MgCl2 and 2.5 μg bovine serum albumin]; Roche Diagnostics, Almere, The Netherlands), 2 pmol each A. lumbricoides-specific primer (Thermo Fisher, Ulm, Germany), 5 pmol each N. americanus-specific primer (Thermo Fisher), 5 pmol each Schistosoma-specific primer (Thermo Fisher), 2.5 pmol each S. stercoralis-specific primer (Thermo Fisher), 1.25 pmol each N. americanus-specific double-labeled probe (Biolegio, Nijmegen, The Netherlands), A. lumbricoides-specific double-labeled probe (Thermo Fisher), S. stercoralis-specific double-labeled probe (Biolegio), and Schistosoma-specific double-labeled probe (Thermo Fisher). Amplification consisted of 15 minutes at 95°C followed by 50 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. Amplification, detection and data analysis were performed with the Corbett Rotor-Gene 6000 Real-Time PCR System (Corbett Research, Mortlake, New South Wales, Australia) and Corbett Rotor-Gene 6000 Application Software, version 1.7.87 (Corbett Life Science, Cambridge, UK). Negative and positive external control samples were included in each amplification run. The details of all primers and detection probes used in our study are described elsewhere.31,32,40,41
Data management and statistical analysis.
The helminth species-specific results derived by each method were entered manually in the participant's CRF and subsequently transferred into a Microsoft Access 2010 electronic database (Microsoft Corporation 2010, Redmond, WA). Data were analyzed using STATA, version 12 (StataCorp., College Station, TX) and R, version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).42
For the comparison of diagnostic methods, the diagnostic results of the first stool sample collected and examined from each participant were included in the analysis. Eligible for inclusion were participants with results on (1) duplicate Kato–Katz thick smears and one FLOTAC dual examination, (2) duplicate Kato–Katz thick smears and one PCR measurement, (3) one FLOTAC dual examination and one PCR measurement, or (4) one Baermann examination and one PCR measurement.
The prevalence of each helminth species investigated is indicated per method and method combination. One must be aware, however, that the participants who submitted stool samples that were included in the present analysis were not a random population sample, because children were recruited partly when they visited a health facility or hospital for asymptomatic, uncomplicated, or severe malaria and because stool samples examined with PCR were selected on purpose and not randomly from individuals who participated in the immunological investigations of the IDEA-malaria study arm. Among 215 stool samples tested with PCR, 123 samples were selected from children who participated in the immunological investigations of the IDEA malaria study arm (i.e., children selected according to their infection status with helminths based on the Kato–Katz thick smear, FLOTAC, and Baermann method results and according to asymptomatic or symptomatic malaria). The additional 92 stool samples were selected randomly from the list of study participants providing stool samples.
Helminth infection intensities were determined by multiplying the species-specific average egg counts from duplicate Kato–Katz thick smears by a factor of 24, dividing the species-specific sum of eggs counted in the two flotation chambers by the measured weight of the preserved stool sample and multiplying the result by factor 1.2 to derive eggs per 1 g stool (EPG). Subsequently, the infection intensity thresholds recommended by the WHO were applied for EPG values derived with the Kato-Katz method.43 The lower limits of moderate and heavy infections were 5,000 and 50,000 EPG for A. lumbricoides, 1,000 and 10,000 EPG for T. trichiura, 2,000 and 4,000 EPG for hookworm, and 99 and 399 EPG for S. mansoni, respectively.
The agreement between the diagnostic methods was assessed using κ-statistics. The κ-statistics were interpreted as < 0.00, poor agreement; 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; 0.81–1.00, almost perfect agreement.44
High PCR cycle threshold (Ct) values reflect low parasite-specific DNA loads and vice versa. In addition to PCR assays, where no amplification curve was obtained, all Ct values above 40 were considered as negative test results.33 To assess if the median of positive Ct values from PCR, the median of positive EPG values derived with the Kato–Katz thick smear method or FLOTAC, or the median of positive larvae counts from Baermann differed between the groups of samples identified as true positives or false negatives with any other method, we used the Wilcoxon rank sum (Mann–Whitney) test. We used a statistical significance level of 5%.
The Pearson correlation was applied to assess an association between PCR Ct values and EPG values derived by the Kato–Katz thick smear method and FLOTAC, respectively, or S. stercoralis larvae counts determined by the Baermann method. In line with codes used in a previous publication about the same topic from another research group,31 PCR assays where no amplification curve was obtained and all Ct values above 40 were considered as negative and coded 45, negative EPG results from duplicate Kato–Katz thick smears were coded 10, negative EPG results from the FLOTAC dual technique were coded 0.1, and negative larvae counts from the Baermann method were coded 0.5.



Two-way contingency table showing the agreement between methods for the diagnosis of hookworm and S. stercoralis infections in stool samples from individuals participating in our study conducted in the United Republic of Tanzania between June of 2011 and November of 2012
| Positive | Negative | Total | |
|---|---|---|---|
| Single FLOTAC | Duplicate Kato–Katz | ||
| Positive | 91 (y11) | 21 (y12) | 112 |
| Negative | 6 (y21) | 1,061 (y22) | 1,067 |
| Total | 97 | 1,082 | 1,179 |
| κ-agreement | 0.86 | ||
| PCR | Duplicate Kato–Katz | ||
| Positive | 40 | 15 | 55 |
| Negative | 15 | 145 | 160 |
| Total | 55 | 160 | 215 |
| κ-agreement | 0.63 | ||
| PCR | Single FLOTAC | ||
| Positive | 43 | 10 | 53 |
| Negative | 17 | 143 | 160 |
| Total | 60 | 153 | 213 |
| κ-agreement | 0.68 | ||
| PCR | Baermann | ||
| Positive | 8 | 9 | 17 |
| Negative | 38 | 138 | 176 |
| Total | 46 | 147 | 193 |
| κ-agreement | 0.14 | ||
The 2 × 2 table was also used for the Bayesian approach (vectors indicated in parentheses) to estimate diagnostic parameters.
Results
Operational results and baseline infections.
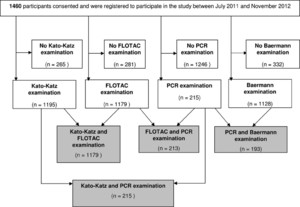
Between July of 2011 and November of 2012, a total of 1,460 participants consented to participate in the screening for helminth infections and inclusion into one of the study arms of the IDEA project (if eligible). Among them, 1,457 and 1,453 had their sex and age recorded, respectively, with 50.3% being male and 49.7% being female; the median age was 5 years (range = 0–98 years). Stool samples of sufficient size for duplicate Kato–Katz thick smears, FLOTAC, and the Baermann method testing were submitted by 1,195, 1,179, and 1,128 individuals, respectively. PCR was applied to 215 stool samples (Figure 1).



Flowchart indicating the number of study participants invited to participate in a helminth screening for the IDEA project in the United Republic of Tanzania between June of 2011 and November of 2012 and the number of stool samples examined with the Kato–Katz thick smear, FLOTAC, Baermann, and PCR methods or a combination thereof for the diagnosis of helminth infections.
Citation: The American Society of Tropical Medicine and Hygiene 90, 3; 10.4269/ajtmh.13-0268
The following overall prevalence values were detected by combining the results from Kato–Katz and FLOTAC testing (N = 1,179): hookworm, 10.0%; T. trichiura, 1.9%; A. lumbricoides, 0.2%; S. mansoni, 0.2%. Applying the Baermann method (N = 1,128), S. stercoralis infections were detected in 7.4% of the participants.
According to the Kato–Katz thick smear method egg count results and WHO thresholds, 84.0% of the hookworm infections were light, 7.0% of the hookworm infections were moderate, and 9.0% of the hookworm infections were heavy. Light and moderate T. trichiura infection intensities were observed in 86.4% and 13.6% of infected participants, respectively. One A. lumbricoides-infected participant had a light intensity of infection, and one A. lumbricoides-infected participant had a moderate intensity of infection. Both S. mansoni infections were light.
Because of the low number of infected individuals, the method comparisons between Kato–Katz and FLOTAC (N = 1,179), Kato–Katz and PCR (N = 215), FLOTAC and PCR (N = 213), and Baermann and PCR (N = 193) were only conducted for hookworm and S. stercoralis infections.
Agreement of diagnostic methods and parameters.
Table 1 shows that the agreement between duplicate Kato–Katz thick smears and the FLOTAC dual technique for hookworm egg detection was almost perfect (κ = 0.86); 21 individuals who were identified as negative by the Kato–Katz method but positive by FLOTAC had a median egg count of 4 EPG (range = 1–430 EPG). Six false-negative results from FLOTAC had a median egg count of 12 EPG (range = 12–24 EPG) in the Kato–Katz thick smears. The median EPG values were significantly lower in the false-negative group than the true-positive group for both methods (Figure 2A and B).



Differences in median hookworm-positive EPG values, median S. stercoralis larvae positive counts, and median positive Ct values in groups of samples identified as true positive or false negative with any other diagnostic method in a study conducted in the United Republic of Tanzania between June of 2011 and November of 2012. *Significant difference (P ≤ 0.05) in the median determined by the Wilcoxon rank sum (Mann–Whitney) test. (A) Difference between hookworm median EPG in true-positive (N = 91) and false-negative (N = 6) FLOTAC samples identified as positive with Kato–Katz (P < 0.001). (B) Difference between hookworm median EPG in true-positive (N = 91) and false-negative (N = 21) Kato–Katz samples identified as positive with FLOTAC (P < 0.001). (C) Difference between hookworm median EPG in true-positive (N = 40) and false-negative (N = 15) PCR samples identified as positive with Kato–Katz (P = 0.438). (D) Difference between hookworm median EPG in true-positive (N = 43) and false-negative (N = 17) PCR samples identified as positive with FLOTAC (P = 0.623). (E) Difference between S. stercoralis median larvae in true-positive (N = 8) and false-negative (N = 38) PCR samples identified as positive with the Baermann method (P = 0.023). (F) Difference between hookworm median Ct values in true-positive (N = 40) and false-negative (N = 15) Kato–Katz samples identified as positive with PCR (P = 0.082). (G) Difference between hookworm median Ct values in true-positive (N = 43) and false-negative (N = 10) FLOTAC samples identified as positive with PCR (P = 0.056). (H) Difference between S. stercoralis median Ct values in true-positive (N = 8) and false-negative (N = 9) Baermann samples identified as positive with PCR (P = 0.194).
Citation: The American Society of Tropical Medicine and Hygiene 90, 3; 10.4269/ajtmh.13-0268
The agreement between PCR and FLOTAC (κ = 0.68) and PCR and Kato–Katz (κ = 0.63) for hookworm diagnosis was substantial; 17 individuals who were not identified as positive by PCR but were identified as positive by FLOTAC had a median egg count of 84 EPG (range = 1–4,603 EPG), and 15 false-negative results by PCR that were detected by the Kato–Katz thick smear had a median egg count of 480 EPG (range = 12–14,064). For both FLOTAC and Kato–Katz methods, the median EPG values in the PCR false-negative group were not significantly lower than in the PCR true-positive group (Figure 2C and D).
A slight agreement (κ = 0.14) was found between PCR and the Baermann method for the detection of S. stercoralis. Thirty-eight individuals with S. stercoralis larvae found by the Baermann method but not PCR had a median of 1 larva identified (range = 1–314 larvae). The median larvae count in the PCR false-negative group was significantly lower than in the PCR true-positive group (Figure 2E).
Correlation between PCR Ct values and microscopic egg/larvae counts.
The median Ct value was 31.4 (range = 24.6–39.3) in the samples with hookworm true-positive egg counts using FLOTAC and 37.8 (range = 26.6–39.2) in false-negative FLOTAC samples. The median Ct value was 31.5 (range = 24.6–39.3) in true-positive Kato–Katz samples and 34.8 (range = 26.6–39.6) in false-negative samples. For both Kato–Katz and FLOTAC methods, there was no significant difference between the median Ct values of the false-negative and true-positive groups (Figure 2F and G). As shown in Figure 3, there was a significant negative correlation between PCR Ct values and hookworm EPG values derived with either FLOTAC (ρ = −0.30; P < 0.001) or Kato–Katz (ρ = −0.36; P < 0.001) methods.



Correlation between hookworm EPG measured with FLOTAC or duplicate Kato–Katz thick smears and Ct values of hookworm real-time PCR in a study conducted in the United Republic of Tanzania between June of 2011 and November of 2012. (A) Correlation between hookworm EPG values measured with FLOTAC and Ct values of hookworm real-time PCR for the detection of N. americanus in fecal samples (N = 211) from coastal Tanzania (Pearson correlation, ρ = −0.30; P < 0.001). (B) Correlation between hookworm EPG values measured with duplicate Kato–Katz thick smears and PCR Ct values of hookworm real-time PCR for the detection of N. americanus in fecal samples (N = 215) from coastal Tanzania (ρ = −0.36; P < 0.001).
Citation: The American Society of Tropical Medicine and Hygiene 90, 3; 10.4269/ajtmh.13-0268
In true-positive and false-negative Baermann samples, the median Ct values were 34.7 (range = 28.6–39.1) and 31.7 (range = 19.7–38.5), respectively. The difference was not significant (Figure 2H). A negative correlation was found between Ct values and the number of S. stercoralis larvae (ρ = −0.14; P = 0.049).
Accuracy estimates of diagnostic methods without pseudo-gold standard.
When directly comparing two methods, the FLOTAC had a significantly higher sensitivity than the Kato–Katz method for detecting hookworm infections (93.8% versus 81.3%; P = 0.006), and the specificity of both methods was almost 100% (Table 2). The sensitivity of the PCR for hookworm infections was equal to the sensitivity of duplicate Kato–Katz thick smears and lower than the sensitivity of FLOTAC. The specificity of the PCR was 93.5% compared with FLOTAC as reference test and 90.6% compared with duplicate Kato–Katz thick smears. The sensitivity of the Baermann method for S. stercoralis detection was significantly higher than the sensitivity of the PCR (47.1% versus 17.4%; P < 0.001). The specificity of the Baermann method was 78.4%, and the specificity of PCR was 93.9%.
Diagnostic accuracy of duplicate Kato–Katz thick smears, FLOTAC dual technique, and real-time PCR for hookworm and the Baermann method and PCR for S. stercoralis detection as well as prevalence according to three different statistical approaches applied in our study conducted in the United Republic of Tanzania between June of 2011 and November of 2012
| Statistical approach | n | Test | Sensitivity, % (95% CI)* | Specificity, % (95% CI)* | McNemar P value† | Prevalence (95% CI)* |
|---|---|---|---|---|---|---|
| Direct method comparison | 1,179 | FLOTAC | 93.8 (87.0–97.7) | 98.1 (97.0–98.8) | 9.5 (7.9–11.3) | |
| Kato–Katz | 81.3 (72.8–88.0) | 99.4 (98.8–99.8) | 0.006 | 8.2 (6.7–9.8) | ||
| Direct method comparison | 215 | PCR | 72.7 (59.0–83.9) | 90.6 (85.0–94.7) | 25.6 (19.9–32.0) | |
| Kato–Katz | 72.7 (59.0–83.9) | 90.6 (85.0–94.7) | 1.000 | 25.6 (19.9–32.0) | ||
| Direct method comparison | 213 | PCR | 71.7 (58.6–82.5) | 93.5 (88.3–96.8) | 24.9 (19.2–31.2) | |
| FLOTAC | 81.1 (68.0–90.6) | 89.4 (83.5–93.7) | 0.248 | 28.2 (22.2–34.7) | ||
| Direct method comparison | 193 | PCR | 17.4 (7.8–31.4) | 93.9 (88.7–97.2) | < 0.001 | 8.8 (5.2–13.7) |
| Baermann | 47.1 (23.0–72.2) | 78.4 (71.6–84.2) | 23.8 (18.0–30.5) | |||
| Combination of methods as gold standard | 212 | PCR | 73.6 (61.9–83.3) | 100‡ | ||
| FLOTAC | 83.3 (72.7–91.1) | 100‡ | ||||
| Kato–Katz | 75.0 (63.4–84.5) | 100‡ | 33.8 (27.5–40.6) | |||
| Combination of methods as gold standard | 1,179 | FLOTAC | 94.9 (89.3–98.1) | 100‡ | ||
| Kato–Katz | 82.2 (74.1–88.6) | 100‡ | 10.0 (8.4–11.9) | |||
| Combination of methods as gold standard | 215 | PCR | 78.6 (67.1–87.5) | 100‡ | ||
| Kato–Katz | 78.6 (67.1–87.5) | 100‡ | 32.6 (26.3–39.3) | |||
| Combination of methods as gold standard | 213 | PCR | 75.7 (64.0–85.2) | 100‡ | ||
| FLOTAC | 85.7 (75.3–92.9) | 100‡ | 32.9 (26.6–39.6) | |||
| Combination of methods as gold standard | 193 | PCR | 30.9 (19.1–44.8) | 100‡ | ||
| Baermann | 83.6 (71.2–92.2) | 100‡ | 28.5 (22.2–35.4) | |||
| Bayesian modeling | 1,179 | FLOTAC | 96.3 (89.3–99.8) | 98.9 (97.6–100) | ||
| Kato–Katz | 89.6 (77.2–99.5) | 99.7 (99.0–100) | 8.9 (7.0–11.0) | |||
| Bayesian modeling | 215 | PCR | 78.8 (1.2–98.8) | 92.7 (3.6–99.6) | ||
| Kato–Katz | 79.2 (1.2–98.8) | 92.8 (3.4–99.6) | 28.1 (17.6–80.1) | |||
| Bayesian modeling | 213 | PCR | 83.3 (64.5–99.1) | 96.2 (90.3–99.8) | ||
| FLOTAC | 88.8 (73.1–99.4) | 93.7 (86.1–99.7) | 26.7 (18.4–36.5) | |||
| Bayesian modeling | 193 | PCR | 11.6 (0.7–89.3) | 90.6 (11.5–99.3) | ||
| Baermann | 28.3 (3.3–95.0) | 75.2 (6.0–96.8) | 43.1 (2.6–97.1) |
95% CI = 95% confidence interval.
In the Bayesian approach the intervals correspond to credible intervals.
P-value for difference in sensitivities determined by the McNemar test on positive individuals.
We assumed 100% specificity.
Accuracy estimates of diagnostic methods using a pseudo-gold standard.
As shown in Table 2, when applying a combination of the available test results (duplicate Kato–Katz thick smears, FLOTAC, and PCR) as a diagnostic pseudo-gold standard, the sensitivity for hookworm diagnosis was highest for FLOTAC (83.3%) followed by Kato–Katz thick smear (75.0%), and PCR (73.6%). For the diagnosis of S. stercoralis, the Baermann method showed a better sensitivity (83.6%) than PCR (30.9%).
Accuracy estimates of diagnostic methods in the absence of a true gold standard using a Bayesian approach.
For the comparison of the FLOTAC and Kato–Katz methods, the two dependence parameters were close to zero, and therefore, we report the following results under the assumption of conditional independence. As shown in Table 2, in the absence of a gold standard, FLOTAC had the highest sensitivity for hookworm detection compared with Kato–Katz thick smear (96.3% versus 89.6%) and PCR (88.8% versus 83.3%). The sensitivities of Kato–Katz thick smear (79.2%) and PCR (78.8%) were estimated to be almost equal. The estimated specificity of the PCR was 96.2% compared with FLOTAC and 92.7% compared with duplicate Kato–Katz thick smears. For the diagnosis of S. stercoralis, both the Baermann method and PCR showed low sensitivity (28.3% and 11.6%, respectively). The specificity of PCR was higher than the specificity of the Baermann method (90.6% versus 75.2%).
Discussion
The upscale of control interventions against neglected tropical diseases over the next years in accordance with the WHO goals set for the year 2020 will likely reduce the prevalence and intensities of soil-transmitted helminth infections in endemic countries. For the decisions of where to implement, when to stop control interventions, and how to implement adequate surveillance to avoid the recrudescence of soil-transmitted helminthiases, sensitive diagnostic methods are needed.52
For the first time to our knowledge, we compared the diagnostic accuracy of the FLOTAC with a previously described real-time PCR assay for hookworm diagnosis, applying three different statistical approaches, and we found that FLOTAC was slightly more sensitive than PCR. When directly comparing each of the techniques with the Kato–Katz method, only FLOTAC and not PCR had a significantly higher sensitivity. The sensitivity of the PCR for S. stercoralis diagnosis was significantly lower than the sensitivity of the Baermann method.
The direct method comparison revealed an equal sensitivity of the PCR and Kato–Katz methods for hookworm diagnosis of 73%. This result of PCR sensitivity is very much in line with findings from another research group that identified sensitivities of 79% and 54%, for PCR and Kato–Katz methods, respectively.53 The considerably higher sensitivity of the Kato–Katz thick smear technique in our study is likely explained by two factors: (i) we performed duplicate and no single thick smears per stool sample per person, and (ii) we examined the Kato–Katz slides exactly after 20 minutes, which avoided the overclearance of hookworm eggs by glycerol. A study conducted in Ghana in 2007 revealed higher sensitivities of 100% and 81% for the PCR and Kato–Katz methods, respectively.31 However, the Ghanaian study participants had higher hookworm infection intensities (median = 720 EPG by Kato–Katz) than our Tanzanian population subsample (median = 516 EPG). A decrease of sensitivity with lower infection intensities has been previously postulated for Kato–Katz and FLOTAC,17,19 and it might also be true for PCR, because Ct values are correlated to the number of eggs detected by the FLOTAC and Kato–Katz methods. The individuals false-negatively diagnosed with PCR who were found positive using Kato–Katz thick smears or FLOTAC, however, did not have significantly lower EPG values than the correctly identified positive individuals, and hence, there must be additional factors that impacted on the sensitivity of the PCR. Inhibition of the PCR by substances present in stool samples might be one possible explanation. Because the external control was always amplified, there might have been stool sample-specific enzymes or other factors that inhibited the DNA amplification in some cases, resulting in false-negative results. The absence of an internal control is a clear limitation of our study. The inclusion of an internal control in each sample (for example, by adding 103 PFU/mL phocin herpes virus 1 into the isolation lysis buffer31) would have shown, if present, that DNA was amplified and hence, if samples were correctly diagnosed as negatives. Another explanation for the non-detection of hookworm positives with PCR might be that the hookworm eggs detected with the Kato–Katz and FLOTAC methods were from A. duodenale, a hookworm species that would not have been identified with the N. americanus-specific primers that we used in our PCR. Only the third-stage larvae of these helminths but not the morphological identical eggs allow a microscopic differentiation between A. duodenale and N. americanus.54 Studies that have undertaken differential diagnosis using coproculture in East Africa have shown that both A. duodenale and N. americanus do occur in East Africa but that the latter is the predominant species in the region.55–57
The lack of accuracy of the PCR for the detection of light helminth infections is reflected in our study with the very low sensitivity (17%, 31%, or 12% depending on statistical approach) of the PCR for S. stercoralis diagnosis and the observation that the group of individuals with false-negative PCR results had a significantly lower median larvae count than the correctly identified positives (1 versus 16 larvae). We found a borderline correlation between Ct values and the number of S. stercoralis larvae detected with the Baermann method, and the PCR was not able to detect all cases and missed light infections. Of note, the PCR sensitivities for S. stercoralis detection were considerably higher in previous studies conducted by other research groups in Ghana (86%)32 and Cambodia (88%)53 compared with the Baermann method, but for example, the median larvae counts in the positive Baermann samples from Cambodia were considerably higher than the median of 1.5 larvae found in our study (F. Schär, personal communication). The specificity of the PCR for hookworm and S. stercoralis in our study was above 90%, regardless of the statistical approach used, and therefore, it is in agreement with other findings.32,53
The higher sensitivity of FLOTAC compared with the Kato–Katz method for the detection of hookworm infection is in line with the results of previous studies.19,20,23,25 The agreement of the two methods was almost perfect. The small group of FLOTAC-tested individuals with false-negative results had a very low median EPG value when they were tested positive with the Kato–Katz method (12 EPG), and also, the group of individuals with positive FLOTAC but false-negative Kato–Katz results had a very low median of 4 EPG. This observation confirms a previous assumption that the sensitivity only drops considerably if the egg counts fall under the lower detection limits of the FLOTAC dual technique (2 EPG) and duplicate Kato–Katz thick smear (12 EPG).58 Also, the PCR was able to identify some cases that were either not detected with the Kato–Katz method and had low EPG values with FLOTAC (N = 5; median = 10 EPG) or were not detected with FLOTAC and had borderline EPG values with the Kato–Katz method (N = 1; median = 12 EPG).
We used three statistical approaches to determine the diagnostic accuracy (mainly to render our results comparable with a set of previously conducted studies, where the sensitivity and specificity of Kato–Katz, Baermann, and PCR methods were determined by direct method comparison31,32,53 or using the combined results of the Kato–Katz and FLOTAC methods as a diagnostic pseudo-gold standard19,20,25). The Bayesian approach was chosen, because it is expected to give a more accurate picture of the true prevalence and might be used in future studies, particularly in light of increased helminth control and elimination efforts, when accurate prevalence estimates will be very important for program decisions. Although the diagnostic sensitivity and specificity and the prevalence estimates for hookworm infections were similar in all three approaches, the sensitivity of the PCR for S. stercoralis infection was estimated considerably lower when using the Bayesian model. The substantial proportion of extra positives identified by either the Baermann method or PCR resulted in this very high estimate of the true prevalence when using the Bayesian model.
Generally, it must be noted that the standardization and adherence to protocols and procedures (particularly for molecular but also for conventional diagnosis of helminth infections) in different laboratories and the implementation of external quality assurance systems would help to render results more readily comparable, evaluate the quality of the PCR and other diagnostic systems used by each respective laboratory, and draw a clearer picture of the sensitivity and specificity of the tests applied for the diagnosis of helminth infections.
We conclude that the diagnostic accuracy of the real-time PCR for hookworm identification is similar to the diagnostic accuracies of the FLOTAC and Kato–Katz methods if the infection intensity is considerably low, which it was in our study. Whether PCR is suitable to give a more accurate picture of hookworm prevalence than the Kato–Katz or FLOTAC method in areas targeted by control interventions against soil-transmitted helminthiases (where infection intensities drop to very low levels) remains to be elucidated. Because Ct values seem to be associated with the number of eggs detected in feces and because the amount of stool used for DNA extraction is small, the currently used PCR protocol might fail to detect very light infections and therefore, cannot readily be applied to monitoring of progressing control programs, confirmation of elimination, or surveillance of disease recrudescence. For these scenarios, innovative diagnostic assays are required that detect very light infections and also, can be performed in a high-throughput format on large population samples to detect a number of parasite species simultaneously. Novel protocols and approaches should be developed that meet these requirements and allow an accurate, standardized, and quality-controlled assessment of the achievements made through intensified helminth control and elimination efforts in light of the WHO goals for the year 2020.
ACKNOWLEDGMENTS
The authors thank all individuals who participated in the IDEA project and all motivated staff from the Bagamoyo Research and Training Centre of the Ifakara Health Institute, Bagamoyo, United Republic of Tanzania, who helped in the field and laboratory with the collection and examination of the samples. We are particularly indebted to Rehema Mussa Mangoli, Tatu Nassoro Matuzya, John Masimba, Kwaba Denis, Shabani Halfani Ally, and Wambura Wanchoke for their great support, commitment, and dedication to work within the IDEA project. Moreover, we would like to thank Professor Giuseppe Cringoli from the University of Naples Federico II, Naples, Italy, for the free provision of multiple FLOTAC apparatuses for this study.
- 1.↑
Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223.
- 2.↑
Christian P, Khatry SK, West KP Jr, 2004. Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet 364: 981–983.
- 3.↑
Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ, 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532.
- 4.↑
Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P, 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288.
- 5.↑
Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P, 2009. Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103: 967–972.
- 6.
Becker SL, Sieto B, Silue KD, Adjossan L, Kone S, Hatz C, Kern WV, N'Goran EK, Utzinger J, 2011. Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis 5: e1292.
- 7.
Knopp S, Steinmann P, Keiser J, Utzinger J, 2012. Nematode infections: soil-transmitted helminths and Trichinella. Infect Dis Clin North Am 26: 341–358.
- 8.↑
Siddiqui AA, Berk SL, 2001. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040–1047.
- 9.↑
Marcos LA, Terashima A, Dupont HL, Gotuzzo E, 2008. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg 102: 314–318.
- 10.↑
Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, Keiser J, Hatz CF, 2012. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142: w13727.
- 11.
Segarra-Newnham M, 2007. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother 41: 1992–2001.
- 12.↑
Schroeder L, Banaei N, 2013. Images in clinical medicine. Strongyloides stercoralis embryonated ova in the lung. N Engl J Med 368: e15.
- 13.↑
WHO, 2012. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases—A Roadmap for Implementation. Geneva: World Health Organization, 1–42.
- 14.↑
London Declaration, 2012. The London Declaration on Neglected Tropical Diseases. Table of Commitments. Available at: http://www.who.int/neglected_diseases/NTD_London_Event_Table_Commitments.pdf. Accessed August 6, 2013.
- 15.↑
Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basanez MG, 2012. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis 6: e1582.
- 16.↑
McCarthy JS, Lustigman S, Yang GJ, Barakat RM, Garcia HH, Sripa B, Willingham AL, Prichard RK, Basanez MG, 2012. A research agenda for helminth diseases of humans: diagnostics for control and elimination programmes. PLoS Negl Trop Dis 6: e1601.
- 17.↑
Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J, 2003. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d'Ivoire. Parasitology 127: 525–531.
- 18.↑
Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J, 2008. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2: e331.
- 19.↑
Knopp S, Rinaldi L, Khamis IS, Stothard JR, Rollinson D, Maurelli MP, Steinmann P, Marti H, Cringoli G, Utzinger J, 2009. A single FLOTAC is more sensitive than triplicate Kato-Katz for the diagnosis of low-intensity soil-transmitted helminth infections. Trans R Soc Trop Med Hyg 103: 347–354.
- 20.↑
Glinz D, Silué KD, Knopp S, Lohourignon KL, Yao PK, Steinmann P, Rinaldi L, Cringoli G, N'Goran EK, Utzinger J, 2010. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis 4: e754.
- 21.
Jeandron A, Abdyldaieva G, Usubalieva J, Ensink JH, Cox J, Matthys B, Rinaldi L, Cringoli G, Utzinger J, 2010. Accuracy of the Kato-Katz, adhesive tape and FLOTAC techniques for helminth diagnosis among children in Kyrgyzstan. Acta Trop 116: 185–192.
- 22.
Ziegelbauer K, Steinmann P, Zhou H, Du ZW, Jiang JY, Fürst T, Jia TW, Zhou XN, Utzinger J, 2010. Self-rated quality of life and school performance in relation to helminth infections: casestudy from Yunnan, People's Republic of China. Parasit Vectors 3: 61.
- 23.↑
Habtamu K, Degarege A, Ye-Ebiyo Y, Erko B, 2011. Comparison of the Kato-Katz and FLOTAC techniques for the diagnosis of soil-transmitted helminth infections. Parasitol Int 60: 398–402.
- 24.
Gualdieri L, Rinaldi L, Petrullo L, Morgoglione ME, Maurelli MP, Musella V, Piemonte M, Caravano L, Coppola MG, Cringoli G, 2011. Intestinal parasites in immigrants in the city of Naples (southern Italy). Acta Trop 117: 196–201.
- 25.↑
Utzinger J, Rinaldi L, Lohourignon LK, Rohner F, Zimmermann MB, Tschannen AB, N'Goran EK, Cringoli G, 2008. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg 102: 84–90.
- 26.↑
de Kaminsky RG, 1993. Evaluation of three methods for laboratory diagnosis of Strongyloides stercoralis infection. J Parasitol 79: 277–280.
- 27.↑
Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, Kita K, Ohtomo H, 1991. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg 45: 518–521.
- 28.↑
Polderman AM, Krepel HP, Baeta S, Blotkamp J, Gigase P, 1991. Oesophagostomiasis, a common infection of man in northern Togo and Ghana. Am J Trop Med Hyg 44: 336–344.
- 29.↑
Yelifari L, Bloch P, Magnussen P, van Lieshout L, Dery G, Anemana S, Agongo E, Polderman AM, 2005. Distribution of human Oesophagostomum bifurcum, hookworm and Strongyloides stercoralis infections in northern Ghana. Trans R Soc Trop Med Hyg 99: 32–38.
- 30.↑
Steinmann P, Zhou XN, Du ZW, Jiang JY, Wang LB, Wang XZ, Li LH, Marti H, Utzinger J, 2007. Occurrence of Strongyloides stercoralis in Yunnan province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis 1: e75.
- 31.↑
Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, Van Lieshout L, 2007. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg 77: 685–690.
- 32.↑
Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, van Lieshout L, 2009. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg 103: 342–346.
- 33.↑
Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, Rahumatullah A, Aziz FA, Zainudin NS, Noordin R, 2011. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg 84: 338–343.
- 34.
Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout L, Petri WA Jr, Haque R, Houpt ER, 2011. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am J Trop Med Hyg 84: 332–337.
- 35.↑
Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER, 2012. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51: 472–480.
- 36.↑
García LS, Bruckner DA, 2001. Diagnostic Medical Parasitology. Washington, DC: American Society for Microbiology, 1–791.
- 37.↑
Katz N, Chaves A, Pellegrino J, 1972. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14: 397–400.
- 38.↑
Cringoli G, Rinaldi L, Maurelli MP, Utzinger J, 2010. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc 5: 503–515.
- 39.↑
Verweij JJ, Pit DS, van Lieshout L, Baeta SM, Dery GD, Gasser RB, Polderman AM, 2001. Determining the prevalence of Oesophagostomum bifurcum and Necator americanus infections using specific PCR amplification of DNA from faecal samples. Trop Med Int Health 6: 726–731.
- 40.↑
Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, van Dam GJ, van Lieshout L, 2008. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol 102: 625–633.
- 41.↑
Wiria AE, Prasetyani MA, Hamid F, Wammes LJ, Lell B, Ariawan I, Uh HW, Wibowo H, Djuardi Y, Wahyuni S, Sutanto I, May L, Luty AJ, Verweij JJ, Sartono E, Yazdanbakhsh M, Supali T, 2010. Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study). BMC Infect Dis 10: 77.
- 42.↑
The R Development Core Team, 2010. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Development Core Team, 1–1731.
- 43.↑
Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L, 1998. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: World Health Organization, 1–48.
- 44.↑
Landis JR, Koch GG, 1977. The measurement of observer agreement for categorical data. Biometrics 33: 159–174.
- 45.↑
Hawass NE, 1997. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol 70: 360–366.
- 46.↑
Joseph L, Gyorkos TW, Coupal L, 1995. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol 141: 263–272.
- 47.↑
Dendukuri N, Joseph L, 2001. Bayesian approaches to modeling the conditional dependence between multiple diagnostic tests. Biometrics 57: 158–167.
- 48.↑
Branscum AJ, Gardner IA, Johnson WO, 2005. Estimation of diagnostic-test sensitivity and specificity through Bayesian modeling. Prev Vet Med 68: 145–163.
- 49.↑
Lunn D, Spiegelhalter D, Thomas A, Best N, 2009. The BUGS project: evolution, critique, and future directions. Stat Med 28: 3049–3067.
- 50.↑
Brooks P, Gelman A, 1998. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7: 434–455.
- 51.↑
Gelman A, Rubin DB, 1992. Inference from iterative simulation using multiple sequences. Stat Sci 7: 457–511.
- 52.↑
Bergquist R, Johansen MV, Utzinger J, 2009. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol 25: 151–156.
- 53.↑
Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S, 2013. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop 126: 89–92.
- 54.↑
Blotkamp J, Krepel HP, Kumar V, Baeta S, Van't Noordende JM, Polderman AM, 1993. Observations on the morphology of adults and larval stages of Oesophagostomum sp. isolated from man in northern Togo and Ghana. J Helminthol 67: 49–61.
- 55.↑
Sturrock RF, 1966. Hookworm studies in Uganda: investigations at Teboke in Lango District. East Afr Med J 43: 430–438.
- 56.
Sturrock RF, 1966. Hookworm studies in Tanganyika (Tanzania): investigations at Hombolo in the Dodoma region. East Afr Med J 43: 315–322.
- 57.↑
Chunge RN, Karumba PN, Andala EO, 1986. Hookworm species in patients from Kenyatta National Hospital Nairobi. Ann Trop Med Parasitol 80: 147–148.
- 58.↑
Knopp S, Speich B, Hattendorf J, Rinaldi L, Mohammed KA, Khamis IS, Mohammed AS, Albonico M, Rollinson D, Marti H, Utzinger J, 2011. Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. PLoS Negl Trop Dis 5: e1036.