BACKGROUND (LASSA VIRUS)
Lassa virus (LASV) is an enveloped, single-stranded, and bisegmented negative-strand RNA virus, belonging to the family Arenaviridae. 1 Lassa fever is a zoonotic disease, primarily transmitted via exposure to feces, urine, or other bodily fluids of the reservoir host, Mastomys natalensis (a multimammate rat). The multimammate rat has been described as one of the most extensive rodent species in West Africa and frequently found in rural homes. Secondary transmission from infected humans to susceptible humans can also occur. 2 Person-to-person (secondary) transmission is possible through direct contact with bodily fluids or surfaces contaminated with the virus. Serological surveys conducted reveal that LASV transmission occurs more frequently in community and hospital settings. 3 A lack of or breakdown in infection prevention and control practices within health facilities propagates nosocomial transmission in endemic countries. There are reports on sexual transmission of the disease; however, studies on the pathophysiology remain limited. 2 The incubation period for LASV ranges between 2 and 21 days. Lassa fever has an overall case fatality rate (CFR) of 1%, whereas patients hospitalized on account of the disease have an estimated CFR of 15%. 2 About 80% of infected patients are asymptomatic or present with mild symptoms. For those who are symptomatic, the clinical manifestation of the viral infection includes fever, malaise, cough, chest pain, difficulty in breathing, abdominal cramps, vomiting, diarrhea, and sore throat. Patients can also present with severe symptoms such as bleeding from mucosal surfaces, pleural effusion, pericardial effusion, seizures, disorientation, and unconsciousness. 4,5
BACKGROUND OF EMERGENCE
Clinical identification and subsequent periodic description of outbreaks in Nigeria.
On the January 12, 1969, a nurse working in a mission hospital in Lassa town, located in the northeastern part of Nigeria, complained of a backache. A week later, the nurse complained of an additional symptom, sore throat. On the 25th of January, she got transferred to a better-equipped mission hospital situated in Jos, Nigeria. Thirty hours post-admission, the nurse died despite the administration of supportive treatment. 6 A staff nurse who worked at the mission hospital where the demised nurse received care complained of flu-like symptoms on the third of February. The presentation was initially misdiagnosed and treated as malaria, an acute febrile illness endemic in Nigeria. 7 She died on the 11th day post-presentation of initial symptoms. A second nurse who worked at the hospital in Jos and assisted with the autopsy of the second case became ill on the 20th of February with similar symptoms as the assumed index and second case. Initially treated symptomatically with an antibiotic (procaine and crystalline penicillin), she received further clinical evaluation and diagnosis in the United States post-transfer. The Yale Arbovirus Research Unit (YARU), New Haven, Connecticut, analyzed the serology samples collected from the three cases. Laboratory analysis of the blood samples at YARU provided strong evidence of a newly discovered virus as the causative pathogen responsible for the array of symptoms and signs observed and elicited in the three cases. 6 A new disease had just emerged, one with the clinical and epidemiological concept not fully understood. Lassa town, known for the first characterized case, got famous for contributing to the name of the disease. Total cases and deaths that occurred during the outbreak were 28 and 13, respectively, accounting for a CFR of 46%. 6
Following the discovery of LASV, outbreaks in Nigeria were reported in Zonkwa (Plateau), Onitsha (Anambra), Vom (Plateau), and Pankshin (Plateau) between 1974 and 1976. Lassa virus reemerged 13 years later in Aboh Mbaise (Aba) and Ekpoma (Edo). Documented outbreaks of the disease were recorded in 17 states between 1990 and 2017. 8 During this period, the highest burden of the disease was in 2012 (1,700 suspected cases and 112 deaths in six states) and 2017 (1,022 suspected cases and 127 deaths in 19 states). 8,9 In 2018, the Nigeria Center for Disease and Control (NCDC) declared another outbreak in the country, involving 23 states. In total, the NCDC reported 3,498 suspected cases, 633 confirmed cases, 20 probable cases, and 2,853 negative cases. The CFR during the outbreak was 27% among confirmed cases. In the same year, seven states reported nosocomial transmission of Lassa fever. In 2019, there was an ongoing outbreak in the country, with 4,500 suspected cumulative cases reported in November. Seven hundred sixty-four (764) cases were confirmed positive, 19 declared probable, and 3,717 reported as negative. Total deaths from the outbreak are 160, accounting for a CFR of 20.9%. The Integrated Disease Surveillance and Response (IDSR) system classifies Lassa fever as an epidemic-prone disease requiring immediate notification. 9
Clinical identification and subsequent periodic description of outbreaks in Liberia.
On March 2, 1972, a woman who was 4 months pregnant got admitted into the obstetric (OB) ward of Curran Hospital, Zorzor, Liberia, on account of threatened abortion. Between admission and discharge, she had an incomplete abortion requiring dilatation and curettage. During the same period, she clinically presented with severe headache, enlarged lymph nodes, facial edema, reduced urinary output, and presence of albumin in her urine. 10 Between the 20th and 26th of March, three patients admitted to the OB ward and seven staff of the hospital all developed symptoms similar to the index case. Collectively, these cases mirrored the documented cases of Lassa fever observed in Lassa in 1969.
Lassa virus was isolated and characterized in four of the serum samples collected from the cases. Two of the cases had viral strains identical to the LASV isolated in Lassa town in 1969. 10 The two outbreaks shared epidemiological resemblance because of exposure to LASV and transmission of Lassa fever (Lf) within healthcare centers. The 1972 outbreak in Liberia had a CFR of 36%. 10 Based on clinical records (showing the clinical presentation of healthcare seekers), it is believed that LASV had been present in Liberia some years before available serological and virological evidence. 11
Clinical identification and subsequent periodic description of outbreaks in Sierra Leone.
A retrospective study with data obtained using clinical information of patients seen at hospitals and meeting the case definition criteria (probable and possible) of Lassa fever was conducted in 1972. The results of the study revealed an ongoing transmission of LASV in the eastern province of Sierra Leone between 1970 and 1972. 12 Although there was evidence (based on clinical, serological, and virological characterization) that LASV was the causative pathogen responsible for the study participants’ manifestations, there was further evidence that demonstrated how the Sierra Leone outbreak was epidemiologically different from the previous outbreaks in Nigeria and Liberia. 12,13 First, Sierra Leone had a protracted outbreak as opposed to the shorter periods experienced in Nigeria and Liberia. Second, the outbreaks documented in 1969 and 1972 were mostly propagated through a person-to-person spread in a hospital setting, whereas cases in Sierra Leone were primarily community acquired. 12 Since its emergence, Lassa fever cases have been seen frequently in the country during the conflict (1991–2002) and post-conflict era. 14
Clinical identification of related viruses in Southern Africa.
In 1977, an arenavirus that was closely related to the LASV was isolated from multimammate rats (M. natalensis) captured in Mozambique, southeast Africa. The arenavirus initially named the “Mozambique virus” (and later given the name Mopeia virus) was immunologically and morphologically related to the LASV discovered about 2,500 miles away from Mozambique. 15 In 1981, immunological antibodies to the Mopeia virus were found in some of the M. natalensis and Aethomys chrysophilus rodents captured in Zimbabwe. This observation revealed the existence of the arenavirus outside the geographical scope of Mozambique, where it had been initially discovered. 16 Although there was no substantial evidence of a previous or ongoing transmission among humans, antibodies to the virus were found in a tested human serum collected from the country. Based on available data at the time, it was suggested that the new arenavirus might be an attenuated antigenic variant of the West African LASV.
Another member of the Arenaviridae family was discovered in 2008 in South Africa. There were five cases of undiagnosed hemorrhagic fever including four who died. 17 The index patient, who became infected from an unidentified source, was airlifted in critical condition from Zambia. Three cases became secondarily infected from the index case, and one case involved the tertiary spread of infection. 18 Laboratory analysis from serum and tissue samples of cases confirmed the presence of a novel virus in the Arenaviridae family. The virus was named Lujo virus in acknowledgment of its geographical source (Lusaka, Zambia, Johannesburg, South Africa). 17 Mobala virus and Ippy virus, members of the “Old World Arenaviruses,” were earlier discovered in the Central Africa Republic (CAR) in 1983 and 1984. Although these arenaviruses share similarities with Lassa fever, their natural hosts are Praomys sp. (soft-furred mouse) and Arvicanthis sp. (grass rats), sequentially. The Mobala virus is predominantly found in CAR, whereas the Ippy virus geographic distribution is the Republic of South Africa. 19,20
Clinical identification and subsequent periodic description of outbreaks in northern Togo.
On the 12th of February, 2016, a 47-year-old expatriate surgeon complained of fever at a private hospital he was conducting clinical work. He was treated with an artemether and lumefantrine drug combination after the malarial tests revealed a positive thick smear. He was also vaccinated against yellow fever. The patient deteriorated despite completing the antimalarial therapy. He subsequently presented with fever, myalgia, chills, headaches, sore throat, and abdominal cramps. 21 Because of deteriorating health conditions, he was flown to Cologne, Germany, for further evaluation, diagnosis, and treatment.
On March 5, 2016, a healthcare staff in Togo who attended to the ill surgeon developed similar flu-like symptoms. He was also treated with the malarial combination drug after a positive thick smear test. 21 The patient deteriorated despite the antimalarial treatment. A blood sample of the second case was sent to the Noguchi Memorial Institute for Medical Research in Ghana and the National Reference Center for Viral Hemorrhagic Fever in France. Lassa virus was confirmed 5 days after the onset of symptoms, and intravenous ribavirin was commenced. A follow-up of 110 contacts (81 healthcare workers (HCWs) and 29 community residents) revealed no additional Lassa fever case. 21
FACTORS CONTRIBUTING TO EMERGENCE AND SPREAD
Public health systems.
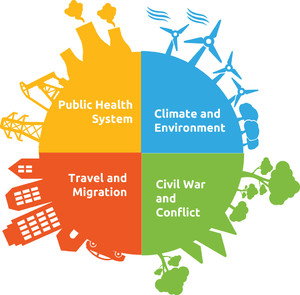
Infectious diseases have emerged and spread in different parts of the world because of an inadequate or lack of a functioning public health system. 22 Deficient public health infrastructure and breakdown of public health measures (Figure 1) have contributed immensely to an unabated emergence and transmission of Lassa fever in West Africa. Some of the components of an optimal public health system include adequate sanitation and hygiene, functioning surveillance system, health education, risk communication, hospital infection prevention and control (as regards nosocomial infection), routine and supplemental immunizations, public health policies and laws, and epidemic preparedness and response. 23,24 A breakdown or lack of any of these components predisposes communities, states, and nations to the late detection of infectious diseases emergence and transmission. Lassa virus was first isolated and characterized in 1969 in Nigeria, an estimated 9-year post-independence. 25 The public health system was in a developmental stage with little country experience to depend on. 26 Also, LASV had never been isolated nor described, making it more challenging to prevent or control. Other countries with Lassa fever outbreaks also share similar public health challenges.



Possible factors contributing to the emergence and spread of infectious diseases in West Africa. 27
Citation: The American Journal of Tropical Medicine and Hygiene 104, 2; 10.4269/ajtmh.20-0487
The overdiagnosis and misdiagnosis of acute febrile illnesses as malaria in sub-Saharan Africa, specifically West Africa, allowed LASV transmission to go undetected for a long time. This misdiagnosis also contributed to an increase in CFR. 21 Malaria is usually the first point of diagnosis in cases presenting with fever. The HCWs treat the patients accordingly. The low index of clinical suspicion and overdiagnosis by HCWs contributed to the transmission of LASV in hospitals and communities in West Africa. 21 Besides prolonging the transmission of the LASV in community and hospital settings, the misdiagnosis of malaria, coinfection with malaria, and delayed diagnosis of Lassa fever worsened patients’ clinical conditions and made control efforts more difficult.
Another significant contributor to the emergence and spread of LASV in West Africa was the lack of an effective public health surveillance system. 28 The IDSR surveillance system was adopted in the WHO African region (AFRO) in 1998. 29 The reemergence of infectious diseases such as cholera, meningitis, yellow fever, and measles in West Africa influenced the initiation of the IDSR strategy. 29 Before the launch of the strategy, countries in Africa (specifically West Africa) had mostly disease-specific control programs. This approach limited the effectiveness of the surveillance system to adequately and promptly detect, notify, and respond to outbreaks of diseases or unusual events. 30 Also, as opposed to the IDSR strategy, which made provisions for surveillance of unusual occurrences/events, prior surveillance systems were designed to address specific diseases. Unusual occurrences of concurrent flu-like illness and hemorrhagic manifestation (as observed in Lassa fever) in the preceding era of the discovery went undetected as a result of the surveillance system. 29 The inadequate surveillance system, in addition to the lack of disease surveillance in wildlife, further contributed to outbreaks and human-to-human spread.
Rodents generally thrive in places with unsanitary and unhygienic conditions. This factor played a role in the emergence and spread of LASV in multiple West African countries. 31 The lack of enforced governmental policies on sanitation and hygiene also influenced the re-emergence of the virus in areas with documented outbreaks. Frequent human and rodent interaction facilitated by the poor sanitary conditions existing at the time played a role in the spillover of the LASV to index cases in the various outbreaks. 32 The multimammate rat (M. natalensis) has been described as one of the most extensive rodent species in West Africa and commonly found in rural homes. This constant interaction and spillover events in these areas have maintained transmission and allowed the disease to be endemic. 33 Some studies have also postulated the role of vertical transmission of LASV from mothers to offspring (in reservoir host) in the reemergence and spread of Lassa fever, although the mechanism is still not fully understood. 33 In addition to rodent-to-human transmission, rodent-to-rodent transmission (horizontal transmission), and rarely, the human-to-rodent transmission, also played some role in Lassa fever endemicity in countries in West Africa. 33
Nosocomial transmission.
The initial outbreaks of Lassa fever in Nigeria, Liberia, and Togo were discovered in healthcare centers. These occurrences signify the role nosocomial transmission plays in the spread of LASV during outbreaks in West Africa. The documented outbreaks that occurred in healthcare facilities suggest an inadequate, or breakdown in infection prevention and control measures. 3,34 Inadequate nursing barrier techniques, insufficient disinfection, indiscriminate use of single needle on multiple patients, inadequate medical equipment sterilizations, and direct/unprotected contact with infected bodily fluids and surfaces were identified as some of the factors driving the spread of LASV within hospitals and secondarily into communities. 34 The lack of funding and support for the public health system within the countries also contributed to the inadequate infection prevention and control precautions. 3
There is also the function of super-spreaders in increasing the transmission rate for LASV within hospitals. A modeling study conducted using Lassa fever as a case study identified the role of super-spreaders in the nosocomial transmission of LASV in the Jos outbreak. 32 Factors contributing to this include the duration of the infectious period, the quantity of LASV expelled, and environmental and social factors. 32 Despite the studies conducted, further research is required to fully understand the role of super-spreaders in sustaining transmission within communities and hospitals.
Effects of civil war/conflict.
Sierra Leone encountered a devastating decade-long civil war from 1991 to 2002. During this period, health facilities were destroyed, and continuous training of health workers was halted. The surveillance system for diseases was adversely affected, leading to no or late detection of Lassa fever cases. Disruption of the country’s disease control programs, infection prevention, and control practices, and health systems collapse facilitated the spread of LASV on reemergence. 35 The post-conflict era also contributed to the re-reemergence of Lassa fever many years after it was first isolated in the country. 14 Between July and September 2002, Lassa fever was diagnosed in 21 peacekeepers within the country (11 Sierra Leone army and 10 UN staff). There is the possibility of a gradually improved public health system postwar era contributing to the detection and subsequent resurgence of Lassa fever cases in the country. Improved surveillance system leads to notification of cases that would typically have gone undetected. Despite the possibility of this, the war and postwar era weakened an already fragile health system. 14 The role of conflicts in the emergence of Lf was observed during the Biafra civil war in the late 1960s. A study of LASV nucleoprotein sequences revealed that the diversity of the viral population found in Nigeria decreased during the civil war between 1967 and 1970, and subsequently stabilized during the last 25 years. 36 This finding buttresses the effect of conflict and war on the emergence and transmission of Lassa fever (Figure 1).
Travel and migration.
The conflicts in Sierra Leone, Liberia, and Nigeria propagated the migration of people from unsafe areas to refugee settlements. As a result of this, migrants were forced into overcrowded camps and unhygienic settlements. The living conditions created an ideal environment for the spread of LASV. 37 The displacement of large populations into unideal settlements increased the exposure of humans to the rodents responsible for LASV transmission. 35 A population genetics analysis conducted in 2012 explored the Lassa fever activity around the Sierra Leone border. The findings revealed that the multimammate rodent population was affiliated with an evolutionary lineage different from the comparison group, the Guinean populations. 36 It was suggested that there was a concomitant migration of the displaced populations and peri-domestic rodent populations from Sierra Leone and Liberia between 2003 and 2005. 36 The analyses also revealed the presence of outbreaks in localities close to refugee areas.
Also, the presence of unwatched and porous borders between countries in West Africa facilitates the emergence and spread of Lassa fever in naive countries. The confirmation of a case of Lassa fever at the Guinea border on the January 15, 2018 was a commendable effort by the public health authorities. 38 However, this indicates the role border surveillance plays in controlling the spread of LASV from endemic countries to countries with no documented outbreaks. Also, over the last decade, cross-country travel has increased, leading to an increased risk of LASV transmission between endemic and non-endemic countries (Figure 2). 37



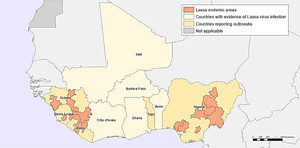
Geographic distribution of Lassa fever cases in West Africa (1969–2018). 39
Citation: The American Journal of Tropical Medicine and Hygiene 104, 2; 10.4269/ajtmh.20-0487
Climate and environment.
Tropical West Africa has a monsoon climate with two major seasons, which are rain (wet) and dry periods. 40 Studies have been conducted to understand the ecology of arenavirus and the relationship between seasonal population dynamics and changes in rainfall. 31,40 Mastomys natalensis rodents breed perennially but have higher fertility during the rainy season. This breeding pattern has been suggested to be due to the effects of rainfall on agricultural practices, leading to an increased availability of food and maturation of crops. 33,41,42, Favorable environmental conditions such as early-onset rain, prolonged rainfall, and increased vegetation cover facilitate an increase in rodents’ reproduction. 41 This seasonal dynamics is a contributor to the emergence and spread in countries in West Africa with documented outbreaks of Lassa fever. 33,43 This evidence is buttressed by a study designed and conducted to describe the risk maps of Lassa fever in West Africa. The results showed that the major single abiotic factor supporting the emergence and spread of Lassa fever is the rainfall pattern. 42
Mastomys natalensis rodents have been known to have an affinity for cereal crops usually grown by subsistence farmers in rural communities in West Africa. The rodents’ forage for grains has created an avenue for frequent interaction between the farmers and the rodents or their bodily secretions/excretions deposited on the agricultural products. 41 These occurrences show the association between rainy seasons and rodents’ population outdoors (around agricultural lands); however, a study conducted in Guinea observed M. natalensis populations were higher inside houses during dry seasons than cultivations nearby. 40 This shift in rodent gatherings and mobilizations was as a result of the food shortage on croplands during dry seasons and the need for the rodents to seek stored crops in homes.
In addition to the effects of seasonal patterns, land-use practices in West African countries have contributed to the emergence and reemergence of Lassa fever. Activities such as the burning of grasslands in preparation for planting season, exploitation of the soil, cultivation of wetlands, application of herbicides to farmlands, and rodents control have played a part in frequently bringing the rodents of interest in close contact with susceptible humans. 33 The interactions lead to LASV spillovers from the reservoirs to humans and subsequently contribute to the emergence and resurgence of human cases in naive populations and populations with previous outbreak documentations.
Social factors.
In addition to the public health system, travel and migration, climatic variation and ecological perturbations, and conflict, human behaviors have substantially played a role in the emergence, reemergence, and propagation of LASV in West Africa. Human activities such as hunting and killing of wildlife M. natalensis rodents have been demonstrated to increase the risk of Lassa fever among exposed individuals. 33 The rodents are used as an alternative and cheap source of protein in the rural and remote communities. The hunting is usually performed in groups of threes and fives, leading to multiple exposures of susceptible individuals. On completion of the hunts for the day, the butchered rodents are carried back to their homes where they are prepared into meals. The preparation predisposes the spouses and children to the bodily fluids of the infected M. natalensis. Although males commonly and majorly practice the hunting activity, both genders are potentially exposed during the whole process (from the hunting to the preparation phase). The hunting and preparation process is a vital factor in the spillover event of LASV from the reservoirs to the index cases during outbreaks. 3 Another sociocultural factor important in the emergence and spread of LASV in West Africa is funeral practices and the myths surrounding the act. 44 It is likewise important to emphasize the part practices such as washing and kissing of dead bodies played in sustaining transmission of LASV in communities. 45,46
PREVENTIVE MEASURES
Preventing and controlling Lassa fever in West Africa requires a holistic approach through the implementation of One Health (Figure 3). This approach is significant in preventing or promptly controlling outbreaks in both endemic and non-endemic countries. First, this section will discuss ways of strengthening existing measures, and second, will discuss the role novel approaches have to play in preventing emergence, reemergence, and the spread of LASV in communities in West Africa.



One Health approach is essential in ensuring a disease-free world. 47
Citation: The American Journal of Tropical Medicine and Hygiene 104, 2; 10.4269/ajtmh.20-0487
Strengthening Lassa fever surveillance and laboratory capacities.
One of the drivers for the late detection and transmission of LASV during the early outbreaks of Lassa fever was the inadequate surveillance system and absence of an integrated disease surveillance system. 28 To prevent outbreaks in endemic countries and to impede the emergence of Lassa fever in naive locations, capacities of the local/district, state, regional, and national surveillance systems must be developed. There must be continuous training of public health workers at the four levels highlighted. Performance indicators such as timeliness and completeness of surveillance reports must be emphasized as one of the core strengths during human capacity development. Another critical aspect absent in countries in West Africa is an effective animal science department and wildlife and domestic animal surveillance. The government of countries in Africa should endeavor to support this adequately.
It is also imperative for the government to invest in local and regional laboratories to complement the community and hospital-based surveillance. The ability of laboratories to isolate pathogens early and notify appropriate public authorities is essential in having an enhanced surveillance system. National authorities must ensure that the IDSR strategy must be fully implemented. Also, the surveillance of rumored outbreaks and uncommon occurrences/events must be incorporated into the indicator-based surveillance system. The role of the Early Warning and Reporting System, as documented in countries using it, must be passed to other countries yet to implement. This initiative should be taken as one of the best practices for other countries to model their warning systems after. 48
Intensification of community awareness and health education.
There is a substantial need for the integration of risk communication, awareness, and health education into primary health care. Generally, communities should be educated on LASV transmission and associated risk factors, but more specifically, emphasis should be placed on populations/communities at most risk of infection. 45,48 Communities should be educated on risk factors such as sociocultural practices (hunting and burial practices), and culturally accepted information, education, and communication (IEC) materials should be used to effect behavioral changes. The IEC messages should be developed using the local language of the geographical locations being targeted.
Water, sanitation, and hygiene advocacy should be incorporated into the health education programs. The provision of clean and affordable water supply by municipal and state governments is essential in allowing communities to practice proper sanitation and hygiene. This sector should be adequately funded, and a structured system put in place to ensure the sustenance. Following the government’s actions, education of communities on proper storage of home food products, washing of hands regularly after contacts with possible contaminants, and maintaining clean homes and surroundings should be effectively communicated.
Infection prevention and control measures.
In endemic areas, most of the documented outbreaks of Lassa fever occurred in healthcare facilities. 3 The occurrence of the outbreaks is an indication of a breakdown in or lack of proper infection prevention and control practices within health facilities. To stop nosocomial transmission of LASV, it is imperative for hospitals and clinics to properly implement and enforce measures that prevent cross-transmission of pathogens from patients-to-patients, patients-to-HCWs, patients-to-caregivers, and vice versa. A proportion of the documented outbreaks during the emergence of Lassa fever reveal the need for proper funding and allocation of required resources. Some of the hospitals have been poorly funded and are situated in underserved/disadvantaged populations. The paucity in funding serves as some of the factors contributing to the nosocomial transmission of LASV. To emphasize—it is not solely appropriate to advocate for needles reuse circumvention, use of nursing barrier techniques, or sterilization techniques utilization—these medical disposables and equipment must be provided in the healthcare facilities. Also, infection prevention officers and hospital epidemiologists, usually missing in some secondary/comprehensive health centers, should be appointed.
Improving access to ribavirin.
The prescription and administration of intravenous ribavirin to patients with Lassa fever does not necessarily play a role in the primary prevention of the disease; however, it assists in reducing the period of infectiousness of patients. The reduction in duration of infectiousness plays a part in reducing the exposure of susceptible populations to the LASV. Ribavirin treatment also assists in reducing the morbidity and mortality of patients with Lassa fever. The effect is more significant when administered within the first 6 days of onset of fever as than in people who received the medication outside this window period. 49 Access to the drug should be improved for populations with limited means.
Increasing access to reproductive care and services.
There have been reports of sexual transmission of Lassa fever. 2 Although there are limited data on the transmission dynamics, reproductive care access should be continuously made available to people in endemic countries and especially to partners of recently recovered Lassa fever patients. Sustainable and simple solutions, such as the use of barrier contraceptives, should be readily accessible. This improved access to critical and supportive services can be achieved with the collaboration between government authorities and partner organizations.
POTENTIAL GAME CHANGERS
Development of new vaccines.
Ribavirin has been shown to reduce the morbidity of patients with Lassa fever; however, the effect is accentuated when administered within 6 days after symptom onset. 49 There is a need for continuous advocacy on the development of vaccines with a differing mechanism of action to primarily prevent Lassa fever in susceptible populations. Lf vaccine development has long been talked about, but the production of a universal vaccine that targets the LASV genetic diversity has been challenging. 50 Despite the demanding situation, progress in the development of vaccines has been made. First, the Coalition for Epidemic Preparedness Innovations (CEPI) launched funding for vaccines in clinical trials in phases 1 and 2. 51 Themis Bioscience, a beneficiary of CEPI’s funding, further tested their vaccine candidate in clinical trials. This is a breakthrough in the fight against Lassa fever globally. Lassa fever is known as a disease of poverty and has a high burden among people with limited resources; therefore, availability of adequate funding for vaccine development is crucial. A Lassa nucleoprotein DNA vaccine being funded by this initiative, INO-4500, has been shown to induce virus-specific CD8+ cells in guinea pigs (during the preclinical phase) and targeted for human trials. 52 Another promising vaccine, a recombinant vesicular stomatitis-based Lassa fever vaccine, was shown to evoke a fast and long-term protective effect in guinea pigs infected with lethal LASV. 53 Notwithstanding, there is a sustained need for additional funding and support from relevant countries and organizations.
Rodent control.
A holistic approach is needed for the prevention and control of Lassa fever. In addition to the development of new vaccines, rodent control is another viable option in the control of the Lf, especially in endemic areas. A chemical rodent control intervention study was conducted in Ghana over a period of 4 years to assess the effectiveness of anticoagulant rodenticides. 31 The intervention was found to be effective in controlling local rodent populations. In addition, trapping of rodent, environmental sanitation, house repairs, and use of rodent-proof storage are measures that can complement the use of rodenticides.
Accelerating development of rapid diagnostic tests.
Lassa fever presents with flu-like symptoms similar to other acute febrile illnesses. Therefore, diagnosing the disease requires a high index of clinical suspicion, and specifically, the use of laboratory testing to confirm the diagnosis. Reverse transcription–PCR (RT-PCR) and ELISA are generally used to assist in viral detection and antigen or antibody detection, respectively. 33 Although the two laboratory techniques have proven to be substantial in laboratory confirmation of Lassa fever diagnosis, their use in rural and remote areas in West Africa remains limited.
The rural areas where the disease remains endemic are often financially deprived. The health centers situated in these areas are poorly funded; therefore, RT-PCR and ELISA are not feasible in this context. There is a need to develop affordable rapid diagnostic kits that can provide a preliminary diagnosis of Lassa fever while awaiting a final confirmation in a reference laboratory. Appropriate organizations should give opportunities to people with creative innovations on Lassa fever diagnosis while also being practical about the use in poor communities. This will also address the issue of underreporting of the disease in some of the health centers. In addition, the rapid diagnosis kits will provide a window opportunity for ribavirin or new antivirals to be administered within a short interval from symptom onset.
Funding development of novel antivirals.
Ribavirin has been effective to a certain degree when administered early to Lassa fever patients. Despite this, there is a need for more funding of new antivirals with mechanism of action directed at specific LASV target sites. Currently, there is a novel drug, LHF-535 (Kineta, Seattle, WA), in a phase 1a trial. It is an enhanced analog of ST-193 (a benzimidazole derivative; SIGA, Corvallis, OR) and acts to inhibit LASV entry by targeting the envelope glycoprotein of the virus. 54 Although drug development is promising, there is an urgent need to fund more research on potential drugs.
CONCLUSION
Lassa fever remains a threat to both endemic and non-endemic countries. To reduce the burden of the disease, it is important to implement strategies that use the One Health approach. Enhanced public health surveillance, further research into development of novel antivirals and vaccines, rodent control, implementing government policies that promote a rebuild of public health infrastructures, and strengthening of fragile health systems are crucial to preventing outbreaks across endemic countries in West Africa. Technical support and funding by countries, institutions, and partner organizations are also essential in eliminating the disease globally.
REFERENCES
- 1.↑
Bowen MD , Peters CJ , Nichol ST , 1997. Phylogenetic analysis of theArenaviridae:patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol 8: 301–316.
- 2.↑
WHO , 2017. Lassa Fever. Available at: https://www.who.int/news-room/fact-sheets/detail/lassa-fever. Accessed November 26, 2019.
- 3.↑
Kernéis S , Koivogui L , Magassouba N , Koulemou K , Lewis R , Aplogan A , Grais RF , Guerin PJ , Elisabeth F , 2009. Prevalence and risk factors of Lassa seropositivity in inhabitants of the forest region of Guinea: a cross-sectional study. PLoS Negl Trop Dis 3: e548.
- 4.↑
Ilori EA et al. 2019. Epidemiologic and clinical features of Lassa fever outbreak in Nigeria, January 1–May 6, 2018. Emerg Infect Dis 25: 1066–1074.
- 6.↑
Frame JD Jr. , Baldwin MB , Gocke DJ , Troup JM , 1970. Lassa fever, a new virus disease of man from West Africa. Am J Trop Med Hyg 19: 670–676.
- 7.↑
Yakubu B , Longdet IY , Tony HJ , Davou DT , Obishakin E , 2019. High-complexity Plasmodium falciparum infections, north Central Nigeria, 2015–2018. Emerg Infect Dis 25: 1330–1338.
- 8.↑
Olayiwola J , Bakarey A , 2017. Epidemiological trends of Lassa fever outbreaks and insights for future control in Nigeria. Int J Trop Dis Health 24: 1–14.
- 9.↑
NCDC , 2018. Nigeria Centre for Disease Control. An Update of Lassa Fever Outbreak in Nigeria. Available at: https://ncdc.gov.ng/diseases/sitreps/?cat=5&name=An%20update%20of%20Lassa%20fever%20outbreak%20in%20Nigeria. Accessed December 19, 2018.
- 10.↑
Monath TP , Mertens PE , Patton R , Moser CR , Pineo L , Gary GW , Kissling RE , 1973. A hospital epidemic of Lassa fever in Zorzor, Liberia, March–April 1972. Am J Trop Med Hyg 22: 773–779.
- 11.↑
Mertens PE , Patton R , Baum JJ , Monath TP , 1972. Clinical presentation of Lassa fever cases during the hospital epidemic at Zorzor, Liberia, March–April 1972. Am J Trop Med Hyg 22: 780–784.
- 12.↑
Fraser DW , Campbell CC , Monath TP , Goff PA , Gregg MB , 1974. Lassa fever in the eastern province of Sierra Leone, 1970–1972. I. Epidemiologic studies. Am J Trop Med Hyg 23: 1131–1139.
- 13.↑
Monath TP , Maher M , Casals J , Kissling RE , Cacciapuoti A , 1974. Lassa fever in the eastern province of Sierra Leone, 1970–1972. II. Clinical observations and virological studies on selected hospital cases. Am J Trop Med Hyg 23: 1140–1149.
- 14.↑
Research Gate , 2014. Lassa Fever in Post-Conflict Sierra Leone. Available at: https://www.researchgate.net/publication/261513808_Lassa_Fever_in_Post-Conflict_Sierra_Leone. Accessed November 28, 2019.
- 15.↑
Wulff H , McIntosh BM , Hamner DB , Johnson KM , 1977. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull World Health Organ 55: 441–444.
- 16.↑
Johnson KM , Taylor P , Elliott LH , Tomori O , 1981. Recovery of a Lassa-related arenavirus in Zimbabwe. Am J Trop Med Hyg 30: 1291–1293.
- 17.↑
Briese T et al. 2009. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever–associated arenavirus from southern Africa. PLoS Pathog 5: e1000455.
- 18.↑
Paweska JT et al. Outbreak Control and Investigation Teams , 2009. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg Infect Dis 15: 1598–1602.
- 19.↑
Gonzalez JP , McCormick JB , Saluzzo JF , Herve JP , Johnson KM , Georges AJ , 1983. An Arenavirus isolated from wild-caught rodents (Praomys species) in the Central African Republic. Intervirology 19: 105–112.
- 20.↑
Swanepoel R , Leman PA , Shepherd AJ , Shepherd SP , Kiley MD , McCormick JB , 1985. Identification of Ippy as a Lassa-fever-related virus. Lancet 1: 639.
- 21.↑
Patassi AA et al. 2017. Emergence of Lassa fever disease in northern Togo: report of two cases in Oti district in 2016. Case Rep Infect Dis 2017: 8242313.
- 23.↑
Davis JR , Lederberg J , 2000. Infections I of M (US) F on E, Introduction. Washington, DC: National Academies Press. Available at: https://www.ncbi.nlm.nih.gov/books/NBK100258/. Accessed November 29, 2019.
- 25.↑
United States, Department of State, Bureau of Public Affairs, Nigeria , 1987. Department of State Publication. Background Notes Series. Washington, DC: Government Printing Office.
- 26.↑
Scott-Emuakpor A , 2010. The evolution of health care systems in Nigeria: which way forward in the twenty-first century. Niger Med J 51: 53–65.
- 27.↑
Butler CD , 2012. Infectious disease emergence and global change: thinking systemically in a shrinking world. Infect Dis Poverty 1: 5.
- 28.↑
Tambo E , Adetunde OT , Olalubi OA , 2018. Re-emerging Lassa fever outbreaks in Nigeria: re-enforcing “One Health” community surveillance and emergency response practice. Infect Dis Poverty 7: 37.
- 29.↑
Fall IS et al. 2019. Integrated Disease Surveillance and Response (IDSR) strategy: current status, challenges and perspectives for the future in Africa. BMJ Glob Health 4: e001427.
- 30.↑
Durski KN , McCollum AM , Nakazawa Y , Petersen BW , Reynolds MG , Briand S , Djingarey MH , Olson V , Damon IK , Khalakdina A , 2018. Emergence of monkeypox — west and central Africa, 1970–2017. MMWR Morb Mortal Wkly Rep 67: 306–310.
- 31.↑
Saez AM , Haidara MC , Camara A , Kourouma F , Sage M , Magassouba N , Fichet-Calvet E , 2018. Rodent control to fight Lassa fever: evaluation and lessons learned from a 4-year study in upper Guinea. PLoS Negl Trop Dis 12: e0006829.
- 32.↑
Iacono GL et al. 2015. Using modelling to disentangle the relative contributions of zoonotic and anthroponotic transmission: the case of Lassa fever. PLoS Negl Trop Dis 9: e3398.
- 33.↑
Gibb R , Moses LM , Redding DW , Jones KE , 2017. Understanding the cryptic nature of Lassa fever in west Africa. Pathog Glob Health 111: 276–288.
- 34.↑
Fisher-Hoch SP , 2005. Lessons from nosocomial viral haemorrhagic fever outbreaks. Br Med Bul 73–74: 123–137.
- 35.↑
Gayer M , Legros D , Formenty P , Connolly MA , 2007. Conflict and emerging infectious diseases. Emerg Infect Dis 13: 1625–1631.
- 36.↑
Lalis A , Leblois R , Lecompte E , Denys C , Meulen J , Wirth T , 2012. The impact of human conflict on the genetics of Mastomys natalensis and Lassa virus in west Africa. PLoS One 7: e37068.
- 37.↑
Richmond JK , Baglole DJ , 2003. Lassa fever: epidemiology, clinical features, and social consequences. BMJ 327: 1271–1275.
- 38.↑
Keïta M et al. 2019. Investigation of a cross-border case of Lassa fever in West Africa. BMC Infect Dis 19: 606.
- 39.↑
Mazzola LT , Kelly-Cirino C , 2019. Diagnostics for Lassa fever virus: a genetically diverse pathogen found in low-resource settings. BMJ Glob Health 4 (Suppl 2): e001116.
- 40.↑
Knippertz P , Fink AH , 2008. Dry-season precipitation in tropical west Africa and its relation to forcing from the extratropics. Mon Weather Rev 136: 3579–3596.
- 41.↑
Massawe AW , Makundi RH , Zhang Z , Mamphi G , Liu M , Li H , Belmain S , 2018. Effect of synthetic hormones on reproduction in Mastomys natalensis. J Pest Sci 91: 157–168.
- 42.↑
Akhmetzhanov AR , Asai Y , Nishiura H , 2019. Quantifying the seasonal drivers of transmission for Lassa fever in Nigeria. Philos Trans R Soc B Biol Sci 374: 20180268.
- 43.↑
Fichet-Calvet E , Lecompte E , Koivogui L , Soropogui B , Dore A , Kourouma , Sylla O , Daffis S , Koulemou K , Meulen J , 2007. Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in Guinea, West Africa. Vector Borne Zoonotic Dis Larchmt N 7: 119–128.
- 44.↑
Kajero O , Del Rio Vilas V , Wood JLN , Lo Iacono G , 2019. New methodologies for the estimation of population vulnerability to diseases: a case study of Lassa fever and Ebola in Nigeria and Sierra Leone. Philos Trans R Soc B Biol Sci 374: 20180265.
- 45.↑
Brown H , Kelly AH , 2014. Material proximities and hotspots: toward an anthropology of viral hemorrhagic fevers. Med Anthropol Q 28: 280–303.
- 46.↑
Wilkinson A , 2017. Emerging disease or emerging diagnosis?: Lassa fever and ebola in Sierra Leone. Anthropol Q 90: 369–397.
- 47.↑
CDC , 2019. Graphics | Multimedia | NCEZID | CDC. Available at: https://www.cdc.gov/ncezid/multimedia/graphics.html. Accessed December 3, 2019.
- 48.↑
Hussain-Alkhateeb L et al. 2018. Early warning and response system (EWARS) for dengue outbreaks: recent advancements towards widespread applications in critical settings. PLoS One 13: e0196811.
- 49.↑
McCormick JB , King IJ , Webb PA , Scribner CL , Craven RB , Johnson KM , Elliot LH , Belmont-Williams R , 1986. Lassa fever. Effective therapy with ribavirin. N Engl J Med 314: 20–26.
- 50.↑
Geisbert TW et al. 2005. Development of a new vaccine for the prevention of Lassa fever. PLoS Med 2: e183.
- 51.↑
Gouglas D , Christodoulou M , Plotkin SA , Hatchett R , 2019. CEPI: driving progress towards epidemic preparedness and response. Epidemiol Rev 41: 28–33.
- 52.↑
NIH , 2019. Safety, Tolerability and Immunogenicity of INO-4500 in Healthy Volunteers - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03805984. Accessed December 2, 2019.
- 53.↑
Purushotham J , Lambe T , Gilbert SC , 2019. Vaccine platforms for the prevention of Lassa fever. Immunol Lett 215: 1–11.
- 54.↑
NIH , 2019. Ascending Oral Dose 14-Day Trial of LHF-535 in Healthy Participants - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03993704. Accessed September 29, 2020.