INTRODUCTION
Sparganosis is a rare parasitic infection caused by the migration of the plerocercoid larvae (sparganum) of tapeworms belonging to the genus Spirometra. Humans are accidental intermediate hosts and can be infected by drinking water contaminated with copepods infected with the procercoid larvae, ingesting raw or inadequately cooked snakes, frogs, or birds infected with the plerocercoids, or applying those meats as a poultice in traditional medicine. The larvae are white, wrinkled, and ribbon-shaped and range from a few millimeters to several centimeters in length. This parasite can be found anywhere in the body and usually presents as a single subcutaneous nodular lesion. 1 Cases of sparganosis are found mainly in China, Japan, Korea, and sporadically in Thailand. Central nervous system (CNS) involvement is sometimes reported with clinical manifestations related to focal brain lesions. Sparganosis of the spinal cord is extremely rare and is most commonly located in the thoracic cord. 2 We herein report a case of spinal sparganosis of the cauda equina, in which the initial diagnosis was a spinal cord tumor, and a review of the literature regarding spinal sparganosis involving the cauda equina.
CASE REPORT
A 54-year-old man presented at the hospital after having experienced lower back pain for 10 months, progressive weakness and numbness of the left leg for 4 months, and urinary incontinence for 3 weeks. This patient had no history of ingesting raw or inadequately cooked snakes or frogs and no presence of peripheral eosinophilia on the laboratory test. Neurological examination revealed mild atrophy of the left lower extremity with motor power of grade IV/V of the proximal part and grade 0/V of dorsiflexion/plantar flexion of the foot, hyporeflexia, decreased pin prick sensation up to the L1 level, and absence of proprioception. Reduced anal sphincter tone and absence of anal reflex were also detected.
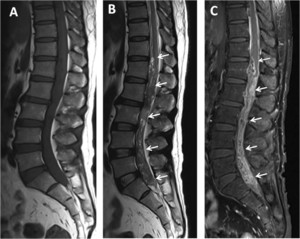
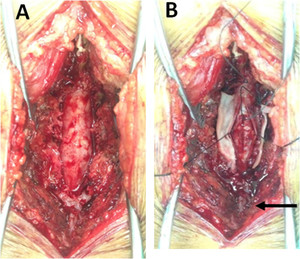
Routine blood tests including full blood count, hematocrit, platelets, liver function test, and renal function tests were unremarkable. Magnetic resonance imaging (MRI) of the thoracolumbosacral spine with gadolinium contrast study showed a 1.2 × 2.3 × 19.8-cm heterogeneous enhancing mass at the T12-S1 level (Figure 1). The initial diagnosis was myxopapillary ependymoma. Laminectomy at L4 and L5 levels was performed, and a matted mass with small cystic lesions in the subarachnoid space and inflamed spinal nerve roots was noted (Figure 2). Partial removal of the matted mass was performed.



Magnetic resonance imaging lumbosacral spines, sagittal plane: T1WI (A), T2WI (B), and gadolinium (GD)-enhanced T1WI with fat suppression (C) showed a long mass-like lesion at the T12-S1 level (arrows). The lesion exhibited isosignal intensity at T1WI, mixed hypo/hypersignal intensity at T2WI, and heterogeneous and irregular enhancement at T1FS with GD. Hypersignal intensity of the spinal cord above the lesion was also detected in the T2WI image (B), which represented diffuse spinal cord edema.
Citation: The American Journal of Tropical Medicine and Hygiene 104, 1; 10.4269/ajtmh.20-0712



Operative field pictures: surgical exposure of the spinal cord, (A) before opening the dura mater, and (B) after opening the dura mater showed enlargement of the thecal sac (A) and a matted lesion with small cysts (arrow) in the subarachnoid space (B).
Citation: The American Journal of Tropical Medicine and Hygiene 104, 1; 10.4269/ajtmh.20-0712
Histological examination revealed a piece of a parasite, surrounded by degenerative connective tissues without specific inflammatory reaction, with a thick bright eosinophilic outer tegument with focal binding and several calcareous corpuscles in a loose stroma, consistent with sparganosis (Figure 3). 3 An immunochromatographic test (ICT) for sparganosis was negative. 4 For molecular identification of the causative parasite species, DNA was extracted from the formalin-fixed paraffin-embedded (FFPE) specimen using a DEXPAT kit (TaKaRa Bio Inc., Tokyo, Japan); the resulting supernatants were used as the DNA template for PCR, and amplification of mitochondrial cytochrome c oxidase subunit 1 gene was performed in a 25-μL reaction mixture, and PCR was accomplished using a GeneAmp PCR System 9700 (Applied Biosystems, Singapore), as reported previously. 4 The PCR fragment was examined using gel agarose electrophoresis. A 467-bp positive band was cut and sent for sequencing at First BASE Laboratories Sdn Bhd (Selangor, Malaysia) using the BigDye terminator v3.1 cycle sequencing kit (ABI). The sequenced result was analyzed using BLAST-N search (National Center for Biotechnology Information, Bethesda, MD), and the causative agent was confirmed to be Spirometra erinaceieuropaei (98.7% similarity to S. erinaceieuropaei, GenBank accession no. AB369251).



Hematoxylin and eosin–stained section: (A) a photograph at ×40 magnification and (B) at ×400 magnification revealed cross sections of a parasitic organism surrounded by degenerative connective tissues without specific inflammatory reaction (A, arrow), with a thick eosinophilic tegumental structure (B, arrow). The subtegumental layer showed several calcareous corpuscles in a loose parenchyma (B, arrowhead).
Citation: The American Journal of Tropical Medicine and Hygiene 104, 1; 10.4269/ajtmh.20-0712
After the operation, the patient received only symptomatic and physical therapy because praziquantel had limited success in CNS sparganosis. 5 However, his neurological symptoms worsened and developed progressive paraparesis. A high-dose regimen of praziquantel (3 × 25 mg/kg body weight daily) and cimetidine (3 × 400 mg daily) was administered for 7 days. 6 At a follow-up 2 years later, the patient’s motor weakness and urinary incontinence had still not improved.
DISCUSSION
The clinical features of cauda equina syndrome are impairment of urinary bladder, bowel, or sexual function, and numbness of the perianal (saddle) area. Other symptoms include back pain, unilateral or bilateral numbness, and weakness of the lower limbs with reduction or loss of deep tendon reflexes. 7 The most common cause is compression arising from large central lumbar disc herniation at the L4/L5 and L5/S1 levels. Other etiologies include congenital conditions, spinal injury with fractures or subluxation, primary or metastatic neoplasms, vascular malformations, aortic dissection, inflammatory arthritis, infections, and iatrogenic causes including manipulation, spinal anesthesia, and postoperative complications. Infectious diseases known to cause this condition are classified into two forms: (1) the primary process directly involving the cauda equina and 2) secondary involvement of the cauda equina, caused by processes of adjunct structures (e.g., vertebral column), and include bacterial infection (e.g., pyogenic pathogens and tuberculosis), fungal (e.g., aspergillosis), viral infection (e.g., varicella-zoster virus and cytomegalovirus), and parasitic infections (cysticercosis, hydatid cysts, schistosomiasis, and sparganosis). 7–9
Spinal sparganosis of the cauda equina has been rarely reported, and to our knowledge, only about six cases have been reported to date in the English literature. 4,10–14 Table 1 summarizes the clinical manifestations and outcomes of these cases and the present case. In four of these seven cases, the patients were male (57.1%). Patients’ age ranges from 26 to 54 years (median: 43.7 years), and the duration of symptoms varied from 1 month to 4 years. All the patients had motor weakness of the lower limbs (one or both legs), and most of them had sensory impairment. Other manifestations were lower back pain and urinary symptoms. These clinical presentations are not distinct from those of any other lesions of the cauda equina. One of the patients had a dual cestode infection (racemose neurocysticercosis). The abnormal MRI findings were heterogeneously serpiginous lesion in five cases (71.4%), bead-like lesion in one case, and nonenhancing cystic lesion in one case. Serologic studies were performed in only three cases, with positive results in two (66.7%). The diagnoses were based on histopathology in all patients. Worms were found at the operative field in two cases (28.6%). A report by Tang et al. 10 suggested that small cystic lesions found during operation were likely due to small trapped cerebrospinal fluid (CSF) spaces in the inflammatory mass.
Clinical features of patients with spinal sparganosis of the cauda equina
| Author | Age/gender, country | Underlying disease | Mode of infection | Clinical presentation and duration of symptoms | Magnetic resonance imaging findings | Serologic method and result | Presence of worms according to operative findings | Surgical treatment | Medical treatment | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Tang et al. 10 | 26/F, China | Thalassemia | Unknown | Lower back, left buttock pain, and numbness in the left leg 1.5 years; urinary incontinence 1 year; left leg weakness 2 months | Mass with coarse heterogeneous hyperintense signal on T2WI and heterogeneous enhancement | Not performed | No | Partial excision | Single dose of praziquantel (5 mg/kg) | Minimal improvement |
| Huang et al. 11 | 26/F. Taiwan | No | Drinking untreated water | General skin rash and itching, followed by lower back pain, paraparesis, hypoesthesia of the left leg, and urinary incontinence 4 months | Mass with heterogeneous enhancement | Not performed | No | No information | No information | No information |
| Boonyasiri et al. 4 | 52/F, Thailand | No | Unknown | Lower back pain 1 month, weakness and numbness of both legs 2 weeks, and bowel/bladder dysfunction 3 days | Heterogeneously serpiginous lesion | ELISA, positive in serum | No | Tissue biopsy | Corticosteroid, praziquantel | Minimal improvement |
| Noiphithak et al. 12 | 54/M, Thailand | Bullous pemphigoid | Ingesting uncooked frogs, snakes, and other amphibians | Lower back pain 2 months, followed by weakness and numbness of the right leg | Hyperintense signal bead-like lesion on T2WI without enhancement | Not performed | Yes | Removal of the worms and lysis of the adhesions | No | Minimal improvement |
| Carlson et al. 13 | 53/M, Taiwan | No | Consuming raw frog and snake meat | Lower back pain 5 months, followed by weakness of the right leg | Nonenhancing, loculated cystic lesion | Not performed | Yes | Removal of the worms | 2-Day course of praziquantel | Marked improvement |
| Chotmongkol et al. 14 | 41/M, Thailand | No | Consuming raw foods | Left hip pain, weakness and numbness of the left leg, and difficulty in urination 4 years | Mixed hypo/hyper signal intensity on T2WI with heterogeneous enhancement | ICT, positive in serum | No | Tissue biopsy | High-dose praziquantel | No improvement |
| Present case | 54/M, Thailand | No | Unknown | Lower back pain 5 months, followed by weakness and numbness of the left leg, and urinary incontinence | Mixed hypo/hyper signal intensity on T2WI with heterogeneous enhancement | ICT, negative in serum | No | Tissue biopsy | High-dose praziquantel | No improvement |
ICT = immunochromatographic test.
In cases such as these, MRI is the optimal method of investigation, as it is both sensitive and noninvasive. The MRI features of spinal sparganosis of the cauda equina are not specific to sparganosis. The differential diagnoses are spinal tumors, such as ependymoma, and other granulomatous inflammations. Interestingly, our review found that the most common abnormal finding in spinal sparganosis of the cauda equina was heterogeneously serpiginous lesions.
The ELISA for anti-sparganum antibodies in the serum or the CSF is a sensitive and specific test to aid in the diagnosis of sparganosis. 15 The iSpa ICT kit (Adtech, Inc., Ltd., Oita, Japan) has been found to be faster and easier to use than ELISA and has shown higher sensitivity and specificity. 16 In cases in which serological tests are negative, PCR-based molecular techniques using FFPE tissues can be beneficial in confirming the diagnosis with species-level identification. 4
The treatment of spinal sparganosis consists of surgical removal of the worms and the granulation tissues, except for cases with severely adhesive lesions to nerve roots within the subarachnoid space, and tissue biopsy is the only way to perform a definite diagnosis. We decided to give praziquantel for this patient because the parasite was only partially removed and symptoms worsened following surgery which could have suggested a still viable parasite. Although a prior study reported successful treatment consisting of high-dose praziquantel therapy for inoperable cerebral sparganosis, 6 the administration of high-dose praziquantel in our patient did not result in any improvement.
In cases of spinal sparganosis, the prognosis has been found to range from fair to good. 2 However, the clinical outcomes of spinal sparganosis of the cauda equina are poor.
CONCLUSION
In this case report, we describe a middle-aged man who presented with cauda equina symptoms (lower back pain, progressive weakness and numbness of the leg, and urinary incontinence). Sparganosis was diagnosed by histological examination and molecular identification of the parasite in the tissue section. Neurological symptoms did not improve after treatment because of the presence of severely matted lesions.
REFERENCES
- 1.↑
Anantaphruti MT , Nawa Y , Vanvanitchai Y , 2011. Human sparganosis in Thailand: an overview. Acta Trop 118: 171–176.
- 2.↑
Kwon JH , Kim JS , 2004. Sparganosis presenting as a conus medullaris lesion: case report and literature review of the spinal sparganosis. Arch Neurol 61: 1126–1128.
- 3.↑
Chung SW , Kim YH , Lee EJ , Kim DH , Kim GY , 2012. Two cases of pulmonary and pleural sparganosis confirmed by tissue biopsy and immunoserology. Braz J Infect Dis 16: 200–203.
- 4.↑
Boonyasiri A , Cheunsuchon P , Srirabheebhat P , Yamasaki H , Maleewong W , Intapan PM , 2013. Sparganosis presenting as cauda equina syndrome with molecular identification of the parasite in tissue sections. Korean J Parasitol 51: 739–742.
- 5.↑
Kim DG , Paek SH , Chang KH , Wang KC , Jung HW , Kim HJ , Chi JG , Choi KS , Han DH , 1996. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg 85: 1066–1071.
- 6.↑
Gonzenbach RR , Kong Y , Beck B , Buck A , Weller M , Semmler A , 2013. High-dose praziquantel therapy for cerebral sparganosis. J Neurol 260: 1423–1425.
- 7.↑
Lavy C , James A , Wilson-MacDonald J , Fairbank J , 2009. Cauda equine syndrome. BMJ 338: 881–884.
- 8.
McNamee J , Flynn P , O’Leary S , Love M , Kelly B , 2013. Imaging in cauda equine syndrome – a pictorial review. Ulster Med J 82: 100–108.
- 9.↑
Batra S , Arora S , Meshram H , Khanna G , Grover SB , Sharma VK , 2011. A rare etiology of cauda equine syndrome. J Infect Dev Ctries 5: 79–82.
- 10.↑
Tang TW , Huang JS , Huang SH , Su KE , Chang YL , 2011. Sparganosis of the spinal canal: rare tapeworm infection as a cauda equine mass with magnetic resonance imaging. J Radiol Sci 36: 139–144.
- 11.↑
Huang CT , Chang MY , Chang CJ , Hsieh CT , Huang JS , 2012. Sparganosis of the cauda equine: a rare case report and review of the literature. Neurol India 60: 102–103.
- 12.↑
Noiphithak R , Doungprasert G , 2016. A case of disseminated central nervous system sparganosis. Surg Neurol Int 7 (Suppl 39): S958–S961.
- 13.↑
Carlson AL , Pruetpongpun N , Buppajarntham A , Damronglerd P , Anderson NW , Apisarnthanarak A , 2017. The brief case: central nervous system sparganosis in a 53-year-old Thai man. J Clin Microbiol 55: 352–355.
- 14.↑
Chotmongkol V , Intapan PM , Jingjit K , 2018. Dual cestode infection in a Thai patient (spinal sparganosis and racemose neurocysticercosis): a case report. Am J Case Rep 19: 1090–1095.
- 15.↑
Kim H , Kim SI , Cho SY , 1984. Serological diagnosis of human sparganosis by means of micro-ELISA. Korean J Parasitol 22: 222–228.
- 16.↑
Yamasaki H , Nakamura T , Intapan PM , Maleewong W , Morishima Y , Sugiyama H , Matsuoka H , Kobayashi K , Takayama K , Kobayashi Y , 2014. Development of a rapid diagnostic kit that uses an immunochromatographic device to detect antibodies in human sparganosis. Clin Vaccine Immunol 21: 1360–1363.