INTRODUCTION
Sri Lanka is an island country in South Asia situated in the Indian Ocean south of India. The country’s tropical climate is influenced by the prevailing ocean winds. Most of the eastern, southeastern, and northern parts of Sri Lanka are more arid, whereas the central highlands and southwestern parts of the country are more humid and get more rainfall.1 Thus, a range of environmental habitats suitable for different animals are present. As in many tropical countries, mosquito-borne diseases were common in Sri Lanka for many decades. However, in 2012, Sri Lanka reported a zero incidence of autochthonous malaria and received WHO certification of its malaria-free status in 2016.2,3 This situation shifted the predominance of vector-borne and zoonotic diseases of concern within the country, resulting in additional attention to the diagnosis of dengue, leptospirosis, and rickettsioses.2
The recognition of scrub typhus, caused by Orientia tsutsugamushi and transmitted by Leptotrombidium mites, in Sri Lanka dates to World War II; it is known to occur in both the hill country and lowlands.4,5 More recently, the frequent occurrence of spotted fever rickettsioses has been demonstrated serologically in both ecozones in Sri Lanka, but particularly in the central hill country.6–9 Depending on the study setting and cohort of the participants, the incidence of spotted fever group (SFG) rickettsiosis-positive individuals ranged from 9.7% among 883 febrile patients in southern Sri Lanka to 63.8% among patients with compatible symptoms in the central province of the country.7,10 Because antigen from African strains of Rickettsia conorii is commonly used for serodiagnostic tests in Sri Lanka, it is sometimes interpreted incorrectly as exposure to R. conorii, as this is a group-specific test.11–15 However, testing of sera of pediatric patients against a panel of eight different SFG rickettsial antigens, and subsequent cross-adsorption and Western blot analysis suggested that exposure to different species of SFG Rickettsia occurs in Sri Lanka.16 The descriptions of Sri Lankan patients with SFG rickettsioses includes some severe clinical manifestations, including the development of acute vasculitis, arthritis, and fern leaf skin necrosis.7,8,17,18 To date, there are only two publications reporting polymerase chain reaction (PCR)-based diagnostic findings in febrile patients from Sri Lanka.18,19 The first article reports detection of the 17-kDa SFG specific protein antigen gene in the skin biopsies of patients with fern leaf necrosis18; however, the specific etiological agent is uncertain because of significant genus-level nucleotide sequence conservation of this gene among SFG rickettsiae.20,21 A second article described a returning traveler from the jungle of Sri Lanka who allegedly experienced a febrile illness with enlarged lymph nodes, a maculopapular rash, and an eschar; this patient tested PCR positive for Rickettsia sibirica mongolotimonae,19 although the sequence of the amplicon was not provided for analysis and comparison. Nevertheless, both reports provide direct molecular confirmation of SFG rickettsioses occurring in Sri Lanka.
People living in the hilly central region in Sri Lanka often sleep on the ground and commonly experience intra-aural tick infestation (otoacariasis), resulting in facial palsies.22 At least four different genera of Ixodid ticks, including Dermacentor, Amblyomma, Rhipicephalus, and Hyalomma species, were found in otoacariasis patients in Sri Lanka.23 These patients exhibited seroconversion to SFG rickettsiae and prompt recovery after doxycycline treatment.22 A country-wide surveillance effort identified as many as 12 Sri Lankan tick species that attach readily to people, and 19 tick species were found on peri-domestic animals.24 Furthermore, 21 tick species were collected from diverse wild animals, including five different Amblyomma species infesting reptiles.24,25 Domestic animals shared tick species typically found on wildlife, suggesting that natural habitat destruction and forest fragmentation may cause wild animals to enter urban and semi-urban neighborhoods, so close contact between wild and domesticated animals can occur. Finally, bovine anaplasmosis resulting from Anaplasma marginale is a serious threat to the cattle industry of Sri Lanka, as in many other tropical and subtropical countries.26 The occurrence of human and canine Anaplasmataceae infections has not been reported in Sri Lanka.
The purpose of our study was to conduct molecular surveillance on ticks collected in different locations in central Sri Lanka to determine which were infected with Rickettsia and Anaplasmataceae. Molecular methods were used to identify the ticks and the agents that were detected.
MATERIALS AND METHODS
Tick collection.
Ticks were collected from different domestic animals that are in close association with the homes and farms of people in Sri Lanka, and by flagging near domiciles upon receiving the owner’s consent. Collection at the Wasgamuwa National Park was done after receiving approval from the local wildlife manager. Additional samples were collected from the animals housed at or brought for examination to the University of Peradeniya Teaching Farm (near Kandy). All ticks were placed in vials containing 70% ethanol and were kept refrigerated. Ticks were examined individually and identified for genus, sex, and life stage using standard taxonomic keys.1,24,27
DNA extraction.
Ticks were surface disinfected though sequential washes with 10% bleach, 70% ethanol, and sterile distilled water; air-dried; frozen in liquid nitrogen; and crushed using sterile Kontes pestles (Kimble-Kontes, Vineland, NJ). The powder was then resuspended in 200 μL nuclei lysis solution (Promega, Madison, WI) supplemented with ethylenediaminetetraacetic acid and proteinase K (QIAGEN, Valencia, CA), and incubated overnight at 56°C. DNA was extracted using a Wizard SV 96 Genomic DNA Purification System (Promega) and a Biomek 2000 Laboratory Automation Workstation (Biomek, Fullerton, CA), as described previously.28 Each DNA was eluted with 100 μL of distilled water and stored at 4°C before testing. Adults and nymphs were processed individually, and larvae were processed in pools (2–11 larvae per pool, depending on their origin and collection site), resulting in a total of 304 DNA samples for further testing.
Molecular identification of ticks.
The ticks were identified primarily by amplification and sequencing a fragment of their 12S ribosomal RNA (rRNA) mitochondrial gene using T1B and T2A primers (Table 1) according to a previously described protocol.29,30 Fragments of COII gene (ticks SL94, SL141, SL148, SL154, SL193, SL199, and SL203) and internal transcribed spacer 2 (ITS2) (SL91, SL94, SL141, SL193, and SL199) were also amplified and sequenced for selected ticks.31 Primer sequences and associated information for individual gene fragments are listed in Table 1.
Primers used in this study
| Target organism | Target gene | Application | Primer name | Sequence 5'-3' | Reference |
|---|---|---|---|---|---|
| Tick | Mitochondrial 12S ribosomal RNA gene | Speciation of ticks (end point PCR) | T1B T2A | AAACTAGGATTAGATACCCT AATAGCGACG GGCGATGT | Beati and Keirans29 |
| COII gene | COIIF COIIR | TCAGAACAYWCYTTYAATCAAAAT CCACAAATTTCTGAACATTGWCCA | Beati et al.31 | ||
| ITS2 | F2LITS2 McLR | TGAGGGTCGGATCAYATATCA GTGAATTCTATGCTTAAATTCAGGGGGT | Beati et al.31 | ||
| SFG Rickettsia | ompA | Testing for SFG rickettsiae (SYBR - Green PCR) | Rr190-547 Rr190-701 | CCTGCCGATAATTATACAGGTTTA GTTCCGTTAATGGCAGCATCT | Eremeeva et al.32 |
| ompA | Speciation of SFG rickettsiae (end point PCR) | Rr190-70 Rr190-701 Rr190-602 | ATGGCGAATATTCTCCAAAA GTTCCGTTAATGGCAGCATCT AGTGCAGCATTCGCTCCCCCT | Eremeeva et al.33 | |
| ompB | 120-M59 120-807 | CCGCAGGGTTGGTAACTGC CCTTTTAGATTACCGCCTAA | Roux and Raoult72 | ||
| sca4 | D1f D928R | ATGAGTAAAGACGGTAACCT AAGCTATTGCGTCATCTCCG | Sekeyova et al.73 | ||
| gltA | RpCS877F RpCS1258R | GGGGACCTGCTCACGGCGG ATTGCAAAAAGTACAGTGAACA | Eremeeva et al.33 | ||
| Anaplasmataceae | 16S rRNA gene | Testing for Ehrlichia and Anaplasma (SYBR-Green PCR) | SYBR-F SYBR-R | AACACATGCAAGTCGAACGG CCCCCGCAGGGATTATACA | Eremeeva et al.,34 Li et al.35 |
| groEL | Speciation of Ehrlichia and Anaplasma (nested PCR) | GRO607F GRO1294R GRO677F GRO1121R | GAAGATGCWGTWGGWTGTACKGC AGMGCTTCWCCTTCWACRTCYTC ATTACTCAGAGTGCTTCTCARTG TGCATACCRTCAGTYTTTTCAAC | Takano et al.36 | |
| Proteobacteria | 16S rRNA gene | Broad-range assay (end point PCR) | Rick16SF1 Rick16SR4 | GTATGCTTAACACATGCAAGTCGAAC TCCGCGATTACTAGCGATTCC | Weisburg et al.37 |
PCR = polymerase chain reaction; rRNA = ribosomal RNA; SFG = spotted fever group.
Detection of Rickettsia DNA.
Individual tick DNA samples were tested using SYBR Green PCR targeting the 547-701-nucleotide (nt) fragment of ompA of the SFG rickettsiae.32 Each 20-μL PCR reaction contained 4 μL of tick DNA, 0.0625 mM final concentration of each forward and reverse primer, 3 mM magnesium chloride, 1 mM deoxynucleoside triphosphate, and 2 μL of 10× SYBR Green Master Mix. DNA from Rickettsia montanensis strain OSU85-930 or R. sibirica strain 246 grown in VERO E6 cells were used as positive controls; sterile distilled water was used as a negative control. All reactions were run for 50 cycles, followed by melting curve analysis of the amplicon. DNA from samples testing positive for the SYBR Green OmpA gene fragment was analyzed further using conventional or semi-nested PCR to amplify longer portions of ompA, ompB, sca4, and gltA according to previously described protocols (Table 1).33 PCR results were evaluated by electrophoresis in 1.2% agarose gels stained with 0.5 μg/mL ethidium bromide.
Detection of Anaplasmataceae DNA.
A portion of the 16S rRNA gene of Anaplasmataceae was detected with a SYBR Green PCR assay and melting curve analysis.34,35 Setup and data acquisition was similar to those described for SFG rickettsiae, with the exception of the specific primers. DNA from Ehrlichia chaffeensis Arkansas grown in the canine macrophage cell line DH82 was used as a positive control; sterile distilled water was used as a negative control. Tick DNA samples testing positive were analyzed further with a nested PCR assay targeting the groEL gene of Anaplasmataceae and broad-range 16S rRNA gene PCR.36,37
Sequencing and sequence analysis.
Individual amplicons, including ompA, ompB, sca4, and gltA fragment genes of Rickettsia, the groEL amplicon of Anaplasmataceae as well as selected tick gene amplicons of the expected sizes were excised from the gel and the DNA was recovered using Wizard PCR Preps according to the manufacturer’s instructions (Promega, Madison, WI). The purified amplicons were sequenced in both directions using PCR primers and the ABI PRISM BigDyeTM Terminator Cycle 3.1 Sequencing kit (Applied Biosystems, Bedford, MA). Sequencing reads were edited and contigs were assembled using Sequencher 5.3 (Gene Codes, Ann Arbor, MI). Primer sequences were removed from assembled contigs, and sequences were analyzed using the National Center for Biotechnology Information (NCBI) Basic Local Alignment Tool (BLAST) search engine. Unique sequences generated during this study were submitted to NCBI GenBank under the following accession nos.: MZ546455-MZ546485, tick 12S mitochondrial rRNA gene; MZ965079-MZ965083, tick ITS2 region; MZ970599-MZ970604, tick COII gene; MZ965077-MZ965078, Coxiella endosymbiont 16S rRNA gene; MZ970589-MZ97059, Ehrlichia and Anaplasma groEL gene; MZ970593-MZ970598, Rickettsia gltA; MZ970572-MZ970588, Rickettsia ompA; MZ970567-MZ970572, Rickettsia ompB; and MZ970562-MZ970565, Rickettsia sca4.
Multiple sequence alignment and phylogenetic analyses.
Multiple sequence alignment and phylogenetic analyses were conducted in MEGA X.38 Each alignment included nucleotide sequences of validated and Candidatus species of Rickettsia or Anaplasmataceae, and the nearest BLAST hits without standing in taxonomy. The evolutionary history was inferred using the neighbor-joining method. The percentages of replicate trees in which the associated taxa clustered together were determined using 500 replicate bootstrap test and are indicated next to the branches. Each tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. All ambiguous positions were removed for each sequence pair (pairwise deletion option).
Statistical analysis.
Statistical analysis was carried out using the Z-test to compare two population proportions, designating a population as individuals that have the characteristic in question. Statistical significance was set at P < 0.05. CIs for prevalence rates in adults and nymphs were calculated using the Wilson score method without continuity correction.39
RESULTS
Tick collection and host associations.
A total of 847 tick specimens from central Sri Lanka were examined as a part of this study, including 94 males, 99 females, 46 nymphs, and 608 larvae (Table 2). Larvae were mostly collected by flagging (n = 603), and were comprised primarily of Amblyomma sp. (99.5%, n = 600) and a few Haemaphysalis and Rhipicephalus. Adult Amblyomma sp. ticks were removed from two tortoises, one pangolin, and three dogs. Rhipicephalus sp. adults and nymphs were mostly from dogs (66%, n = 118 Rhipicephalus from animals) and cattle (33%). Haemaphysalis sp. adults and nymphs were mostly from cattle (77%, n = 101 of Haemaphysalis from animals), whereas the remaining few samples were from dogs, goats, and pigs.
Summary of ticks examined, their locations in Sri Lanka, and vertebrate hosts
| Location | Tick genus | Host (source) | Ticks, n | Male, n | Female, n | Nymphs, n | Larvae, n | Positive for SFG | Positive for Ehr/An | Molecular identification |
|---|---|---|---|---|---|---|---|---|---|---|
| Hemmathagama | Haemaphysalis | Flagging | 4 | 0 | 0 | 2 | 2 | 0 | 0 | – |
| Cow (2) | 17 | 8 | 8 | 1 | 0 | 4 | 0 | – | ||
| Dog (1) | 2 | 2 | 0 | 0 | 0 | 0 | 0 | – | ||
| Rhipicephalus | Flagging | 1 | 0 | 0 | 0 | 1 | 0 | 0 | – | |
| Cow (1) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | – | ||
| Dog (2) | 3 | 3 | 0 | 0 | 0 | 2 | 0 | R. massiliae-like (SL148) | ||
| Goat (1) | 2 | 2 | 0 | 0 | 0 | 1 | 1 | R. kellyi (SL154) | ||
| Galaha | Haemaphysalis | Goat (3) | 5 | 0 | 1 | 4 | 0 | 0 | 1 | – |
| Rhipicephalus | Cow (2) | 28 | 10 | 18 | 0 | 0 | 1 | 7 | Ehrlichia sp. (SL193|SL199) | |
| Gambola | Amblyomma | Dog (1) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | – |
| Haemaphysalis | Pig (1) | 6 | 0 | 0 | 1 | 5 | 1 | 0 | – | |
| Rhipicephalus | Dog (2) | 2 | 2 | 0 | 0 | 0 | 0 | 1 | – | |
| Minuwangoda | Haemaphysalis | Cow (2) | 47 | 5 | 39 | 3 | 0 | 0 | 3 | – |
| Dog (2) | 10 | 7 | 2 | 1 | 0 | 0 | 1 | – | ||
| Rhipicephalus | Dog (3) | 24 | 7 | 5 | 12 | 0 | 0 | 4 | – | |
| Peradeniya Farm | Amblyomma | Monkey (2) | 5 | 5 | 0 | 0 | 0 | 0 | 0 | – |
| Pangolin (1) | 4 | 4 | 0 | 0 | 0 | 0 | 1 | Ehrlichia sp. (SL91) | ||
| Haemaphysalis | Cow (4) | 14 | 0 | 6 | 8 | 0 | 1 | 0 | – | |
| Rhipicephalus | Cow (1) | 1 | 0 | 0 | 1 | 0 | 1 | 0 | R. massiliae-like (SL261) | |
| Dog (1) | 3 | 2 | 0 | 1 | 0 | 1 | 0 | R. massiliae-like (SL275) | ||
| Wasgamuwa Park | Amblyomma | Flagging | 600 | 0 | 0 | 0 | 600 | 45 | 2 | R. africae (8 larva pools) |
| Dog (2) | 2 | 0 | 1 | 1 | 0 | 1 | 0 | – | ||
| Tortoise (2) | 8 | 7 | 1 | 0 | 0 | 1 | 1 | Anaplasma sp. (SL103) | ||
| Rhipicephalus | Cow (1) | 9 | 5 | 4 | 0 | 0 | 0 | 0 | – | |
| Dog (7) | 48 | 24 | 13 | 11 | 0 | 7 | 0 | R. massiliae (SL141|SL277), Rickettsia Pakistani type (SL94) | ||
| Total | Ticks from 3 genera | – | 847 | 94 | 99 | 46 | 608 | 65 (7.7%) | 23 (2.7%) | – |
Ehr/An = Ehrlichia and Anaplasma; SFG = spotted fever group rickettsiae.
Analysis of 12S mitochondrial rRNA fragment sequences identified dog-infesting Rhipicephalus ticks as R. haemaphysaloides (98% of sequence identity to NCBI accession no. MW080207) and the tropical lineage of Rhipicephalus sanguineus (99% of sequence identity to AY559842). Rhipicephalus ticks removed from cattle were identified as Rhipicephalus annulatus (99% sequence identity to EU921773). The 12S mitochondrial rRNA fragment sequences generated for Haemaphysalis ticks were similar to each other and were related most closely but were not identical to a homologous sequence of the 12S mitochondrial rRNA fragment from Haemaphysalis flava (90% of sequence identity to JF58621) and Haemaphysalis longicornis (90.62% of sequence identity to MK450606). The 12S mitochondrial rRNA fragment sequences from adult Amblyomma ticks removed from tortoise and pangolin differed from each other and represented two unique genotypes that do not have significant matches to homologous sequences from other Amblyomma species available from the NCBI GenBank database. The third 12S mitochondrial rRNA fragment sequence genotype was detected in Amblyomma larvae, and this sequence also did not match any existing sequences searchable by BLAST though the NCBI GenBank. Because the existing database of sequences for the COII tick gene and the ITS2 spacer region fragment is even less robust, sequencing these fragments for Sri Lankan ticks did not contribute to their specific molecular identification.
Testing ticks for SFG rickettsiae.
Sixty-five of 304 tick DNA samples tested positive using OmpA gene SYBR Green assay (21.4%; 95% CI, 17.1–26.) (Table 2). Most of these positive samples were DNA from 62 pools of Amblyomma larvae; 45 pools tested positive, with an estimated minimum infection rate of 7.5% (95% CI, 5.6–9.9). Only 2 of 20 adult Amblyomma tick DNAs tested positive for the OmpA gene (10%; 95% CI, 2.8–30.1). Thirteen of 118 Rhipicephalus tick DNAs tested positive for the OmpA gene (11.02%; 95% CI, 6.6–17.9). SFG Rickettsia DNA was detected at a similar rate in Haemaphysalis tick DNAs when compared with Rhipicephalus tick DNAs (z = –1.4947, P < 0.05), as it was present in 6 of 100 DNAs tested (6%; 95% CI, 2.8–12.5). SFG rickettsiae belonging to five different genotypes were identified in these samples using multiple-locus sequencing.
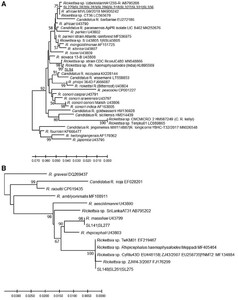
Sequences of amplicons of the 70-602-nt OmpA gene fragment from DNA from eight Amblyomma larval pools (SL279, SL282, SL283, SL286, SL318, SL327, SL331, and SL336) were identical to each other and had significant sequence similarity (99%, three single nucleotide polymorphisms [SNPs]) to the homologous fragment from Rickettsia africae ESF5-type strain (U43790) from Amblyomma variegatum from Ethiopia. There was one A|G SNP at the 23rd nucleotide, which resulted in a Q|R-predicted amino acid mutation in the corresponding sequence of the R. africae detected in Amblyomma larvae from Sri Lanka. Phylogenetic analysis based on the ompA fragment placed these Sri Lankan rickettsiae in a separate lineage from the ESF5 isolate, together with other molecular isolates of R. africae (one SNP difference only) detected in other ticks across broad geographic regions including India (Haemaphysalis larvae), Uzbekistan (Hyalomma aegypticum), Zambia (unspecified tick), and from Egypt (two SNP differences) (H. Hyalomma dromedarii and Hyalomma impeltatum) (Figure 1A and Supplemental Figure S1).



Sub-trees demonstrating (A) the genetic relationship of Rickettsia ompA genes from Amblyomma larval pools and SL94 Pakistani-like isolate, and (B) the genetic relationship of Rhipicephalus massiliae and R. massiliae-like isolates detected in Sri Lankan ticks. Samples of Sri Lankan ticks are indicated by the letters SL and corresponding tick number, and are underlined. The evolutionary history was inferred using the neighbor-joining method computed using the Kimura 2-parameter method in MEGA X.38 This analysis involved 57 nucleotide sequences of validated and Candidatus species of Rickettsia, and the nearest Basic Local Alignment Tool hits of Rickettsia without standing in taxonomy. There were 540 positions in the final data set.
Citation: The American Journal of Tropical Medicine and Hygiene 106, 6; 10.4269/ajtmh.21-0995
Amplicons of the 70-602-nt OmpA gene fragment each derived from the DNAs of R. haemaphysaloides ticks from two different dogs (SL141, SL277) from the Wasgamuwa site were identical to each other and had 100% sequence similarity with a homologous OmpA gene fragment of Rhipicephalus massiliae Mtu5. Three other R. haemaphysaloides from dogs and cattle (SL148, SL261, SL275) from two different locations yielded an OmpA gene fragment with a nucleotide sequence with the greatest similarity to R. massiliae among Rickettsia with a recognized species status (98%). There were six SNPs and a 3-nt INDEL in Rickettsia sequences from Sri Lanka, resulting in six amino acid differences from R. massiliae Mtu5. OmpA fragment sequences (SL148, SL261, SL275) identical to those detected in the ticks from Sri Lanka have been detected previously in Rhipicephalus turanicus from Cyprus, R. haemaphysaloides from deer (described as an endosymbiont) in India and Taiwan (TwKM01 EF219467), and an unidentified cattle-biting tick in Yunnan Province, China (Figure 1B and Supplemental Figure S1). This agent was confirmed to be closely related to TwM01 by sequencing ompB and sca4 fragments from SL148 and SL275 ticks. A similar but not identical agent called Rickettsia sp. SriLankaAT31-R has been detected recently in Amblyomma trimaculatum found on snakes (Boiga forsteni) from one of the breeding facilities in Sri Lanka.40
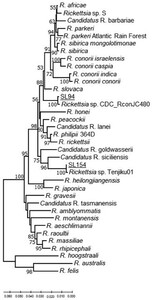
The nucleotide sequence of a 111-nt small OmpA gene fragment amplified from R. haemaphysaloides (SL154) removed from a goat in Hemmathagama had 100% identity to the sequence from a homologous fragment from uncultured Candidatus R. kellyi (DQ08005.1) (Supplemental Figure S2). Unfortunately, further attempts to amplify a larger portion of the OmpA gene failed. However, larger fragments of the OmpB gene and gltA were amplified from the same tick DNA and thus presumably from the same SFG Rickettsia. Analysis of these two concatenated gene fragments showed that this SFG Rickettsia represents a unique lineage within the SFG rickettsiae that is most related to the uncultured Candidatus R. siciliensis identified previously in R. turanicus from Sicily41 (Figure 2 and Supplemental Figure S1). The OmpA gene fragment sequence of Candidatus R. siciliensis has a 93.88% sequence identity with Rickettsia sp. CMCMICRO2 (HM587249), detected in a febrile patient and four other similar cases (HM587248, HM587250, HM587251, HM587253) in southern India.42 These are almost 99% identical to the small ompA sequence fragment listed for Candidatus R. kellyi43 and 100% to Rickettsia sp. Tenjiku01 (LC089865) associated with a travel case to India.44 Thus, it appears these four agents are closely related (Supplemental Figure S2).



Genetic relationships of Candidatus R. kellyi (SL154) and Rickettsia SL94 detected in Sri Lanka based on concatenated gltA–ompB fragments. Samples of Sri Lankan ticks are indicated by the letters SL and corresponding tick number, and are underlined. The evolutionary history was inferred using the neighbor-joining method computed using the Kimura 2-parameter method in MEGA X.38 This analysis involved 38 nucleotide sequences of validated and Candidatus species of Rickettsia, and the nearest Basic Local Alignment Tool hits of Rickettsia without standing in taxonomy. There were 1,147 positions in the final data set.
Citation: The American Journal of Tropical Medicine and Hygiene 106, 6; 10.4269/ajtmh.21-0995
The OmpA gene fragment sequence (SL94) from a R. haemaphysaloides found on a dog in Wasgamuwa Park had the greatest sequence similarity to the homologous ompA fragment of Rickettsia slovaca (95%). Moreover, the high sequence homology is shared with a SFG Rickettsia detected in a Rhipicephalus sp. tick from Pakistan (Rickettsia sp. CDC_RconJC480 MN548866) and a R. haemaphysaloides tick from southern India (KU895509) (Figure 1A and Supplemental Figures S1 and S2). Further analysis of the concatenated fragments of gltA and ompB (Figure 2), and gltA-sca4-ompB (Figure 3) indicated that this SFG Rickettsia represents a new genetic type among currently known Rickettsia.



Genetic relationship of Rickettsia SL94 detected in Sri Lanka based on concatenated gltA–sca4–ompB fragments. Samples of Sri Lankan ticks are indicated by the letters SL and corresponding tick number, and are underlined. The evolutionary history was inferred using the neighbor-joining method computed using the Kimura 2-parameter method in MEGA X.38 This analysis involved 36 nucleotide sequences of validated and Candidatus species of Rickettsia, and the nearest Basic Local Alignment Tool hits of Rickettsia without standing in taxonomy. There were 2,099 positions in the final data set.
Citation: The American Journal of Tropical Medicine and Hygiene 106, 6; 10.4269/ajtmh.21-0995



groEL gene fragment genetic relationship of Ehrlichia and Anaplasma detected in ticks from Sri Lanka. Samples of Sri Lankan ticks are indicated by the letters SL and corresponding tick number, and are underlined. The evolutionary history was inferred using the neighbor-joining method computed using the Kimura 2-parameter method in MEGA X.38 This analysis involved 34 nucleotide sequences. There were 322 positions in the final data set.
Citation: The American Journal of Tropical Medicine and Hygiene 106, 6; 10.4269/ajtmh.21-0995
Testing ticks for Anaplasmataceae.
Twenty-three DNA samples tested positive for the Anaplasmataceae 16S rRNA gene fragment by SYBR Green PCR. Direct sequencing of the small PCR amplicon confirmed that the DNA detected belongs either to Ehrlichia or Anaplasma. Sequencing and analysis of the groEL fragments determined that the Amblyomma sp. tick (SL103) removed from a tortoise in Wasgamuwa was infected with an Anaplasma that clustered together with various Anaplasma identified in association with livestock animals, but also with wild animals including reptiles. However, the genetic similarity was low, with the greatest at 80.82% similarity with a homologous RT4 groEL fragment detected in Amblyomma varanense removed from a water monitor, Varanus salvator, in Indonesia (LC428380). One Amblyomma tick (SL91) removed from a pangolin brought to Peradeniya Farm tested positive for a groEL fragment that had 91.3% (U13638) to 93.2% (CP033456) sequence similarity with various isolates of Ehrlichia ruminantium. Two R. annulatus (SL193, SL199) removed from a cow in Galaha tested positive for a groEL fragment of an Ehrlichia sp. That is related to Ehrlichia ewingii (92.2%, AF195273); however, it clearly represents a unique lineage within that Ehrlichia cluster. Of note, this or closely related groEL genotypes were found previously in Rhipicephalus microplus from Malaysia (KY046306) and Guinea (MW054558), and an unknown tick from Yunnan Province, China (KY705065); consequently, this Ehrlichia organism may circulate widely.
Analysis of the 16S rRNA gene amplicons derived from the tick DNA using the broad-range PCR Weisburg’s primers did not contribute to deciphering more completely the nature and genotype of the Ehrlichia and Anaplasma agents identified based on groEL fragment sequences. The amplicons of most ticks were from Morganella and Elizabethae Elizabethkingia meningoseptica; however, several ticks (SL91, SL157) yielded amplicons matching those of a Coxiella endosymbiont detected previously in various species of Haemaphysalis and less frequently in Rhipicephalus ticks.
DISCUSSION
Increasing numbers of reports of tick-transmitted rickettsial diseases in Sri Lanka have been published in the past two decades.7–10,16,18 These autochthonous cases occurred in diverse populations across the country. Traditionally, Rickettsia conorii indica was associated geographically with cases of spotted fever rickettsioses on the Indian subcontinent and Sri Lanka,45 and recently its etiological role has been confirmed in northern India.46 Consequently, most cases of suspected spotted fever rickettsiosis are diagnosed serologically as R. conorii infections using homologous antigens.11–14 However, at least one serological study used a cross-adsorption IFA protocol and concluded that exposure to more than one SFG rickettsiae may occur in Sri Lanka.16 This observation is in agreement with descriptions of three cases of travel-acquired rickettsioses in tourists returning from Sri Lanka and India to Australia, France, and Japan, indicating that R. conorii is probably not the sole etiological agent in Sri Lanka,19,44,47 We performed testing of ticks obtained in the vicinity of human dwellings and collected from peri-domestic animals to evaluate the presence of rickettsial agents posing potential risks to humans. The samples were mostly from cows and dogs, which can serve as sentinels of human exposure to tick-borne rickettsioses.48 Prior canine serological surveillance determined antibodies to SFG in 42% of dogs (n = 123) from Rajawatta, Thambavita, and areas of the western slopes and Unawatuna of Sri Lanka.49
The ticks we examined included three different types of Amblyomma ticks, one species of Haemaphysalis ticks, and at least three species of Rhipicephalus ticks based on sequencing the 12S mitochondrial rRNA gene fragment. Consequently, our survey must be considered only an initial snapshot of the tick-borne rickettsial agents found in Sri Lanka. Expanded tick collections (geography, temporal, and hosts), improved morphological keys for immature ticks, and a detailed molecular/morphological database are necessary to clarify the range of ticks in Sri Lanka that harbor rickettsial agents. Nonetheless, five different genotypes of SFG rickettsiae were identified in our sample of ticks: 1) a R. africae-like agent in Amblyomma larvae, 2) R. massiliae and a related genotype identified in association with R. sanguineus from dogs, 3) R. haemaphysaloides from dogs and cattle, 4) Candidatus R. kellyi, and 5) another novel genotype in R. haemaphysaloides. Rickettsia massiliae is known to be an infrequent human pathogen that may cause a severe form of rickettsiosis manifesting with typical eschar and purpuric or maculopapular rash, and bilateral chorioretinitis.50,51 Pathogenicity of the other closely related R. massiliae-like genotype is currently unknown; however, similar to R. massiliae, it appears to be broadly distributed worldwide based on an existing publication52 and many NCBI GenBank submissions of unpublished findings.
Rickettsia africae, the etiologic agent of African bite fever, is commonly vectored by Amblyomma sp. ticks in sub-Saharan Africa and the West Indies.45 Use of molecular tools expanded our current knowledge and findings of R. africae to northern Africa and middle eastern to western Asia, as well as its presence as a divergent clade in various species of Hyalomma, Haemaphysalis, and Rhipicephalus ticks.45 Interestingly, R. africae strains circulating in different geographic locations are not homogeneous because they exhibit genetic heterogeneity of the OmpA, OmpB, and Sca4 gene fragments used for genotyping.53 This suggests ongoing evolution and diversification of this widespread lineage of Rickettsia. We demonstrate that the divergent clade can also occur in different Amblyomma species in Sri Lanka. The impact of this process on the biological properties of R. africae and its role in the prevalence of human and animal exposures to rickettsiae and contribution to Rickettsia-caused diseases remain unknown. Because we found it only in ticks at one location and eschars, a common feature of classic African tick-bite fever reported rarely in this country, this Rickettsia may be introduced only recently to the island either with migratory birds or livestock.54,55 Nevertheless, local physicians should consider the diagnosis of African tick-bite fever in residents of Sri Lanka without a travel history to endemic areas for this agent.
Candidatus R. kellyi was first identified as an etiological agent in a pediatric patient from Thiruppathur, Tamil Nadu, India, who experienced a febrile illness with a maculopapular rash43; additional patients were identified subsequently in the same part of India.42 Furthermore, a new SFG rickettsiosis provisionally called Candidatus R. indica Tenjiku01 was diagnosed in a Japanese traveler returning from India,44 and this Rickettsia appears to be the same as Candidatus R. kellyi. Detection of Candidatus R. kellyi sequences (SL154) in our investigation indicates its presence in Sri Lanka, and it also provides the first record of the likely tick vector (R. haemaphysaloides) and an associated animal host (goat) involved in natural maintenance of this pathogen. It should be noted that R. haemaphysaloides is collected frequently from dogs and goats in Sri Lanka, thus suggesting that diverse peri-domestic animals may be a part of this tick’s natural cycle; this tick can also attach to people readily and then transmit rickettsiae during feeding.24
Another genotype of SFG rickettsiae (SL94) was also detected in R. haemaphysaloides collected from a dog. Genetic sequences of SL94 exhibited the greatest similarity to the yet-unnamed Rickettsia CDC_RconJC480 that was first isolated from a pool of Rhipicephalus sp. nymphs removed from Nesokia indica (short-tailed bandicoot rat) in West Pakistan.56 The original isolation was done in guinea pigs, and seroconversion and microscopic detection of Rickettsia-like organisms were used as a diagnostic tool to suggest it was related to R. conorii; however, seroconversions in guinea pigs were not commonly associated with any recognizable signs of infection. Subsequent typing using a so-called rickettsial toxin neutralization test in mice also identified RconJC480 as a strain of R. conorii.57 These findings preceded the recognition of R. slovaca as a human disease agent.58 More recent multilocus typing analysis using conventional rickettsial genes (ompA, sca4, gltA, the 17-kDa antigen gene, and ompB), additional variable Rickettsia genes (atpA, virB4, dnaA, dnaK, and recA), and four informative intergenic spacer region sites (rrl-rrf, dksA-xerC, mppA-purC, and rpmE-tRNA-fMet) indicated that RconJC480 represents a unique lineage within the SFG Rickettsia (G.A. Dasch, personal communication and corresponding sequences submitted to GenBank). Our analysis corroborates these observations and conclusions. A similar SFG Rickettsia has been detected in R. haemaphysaloides in Kerala state, India, thus further suggesting the role of R. haemaphysaloides in circulation of this Rickettsia. It is currently unknown whether RconJC480 is a human pathogen.
To the best of our knowledge, this study is the first effort to conduct direct molecular detection and identification of Anaplasmataceae in ticks from Sri Lanka. Three different types of Anaplasma and Ehrlichia were found in the ticks tested; however, it should be emphasized that only preliminary identification was completed for these organisms, and it was based on a relatively short fragment of the conserved groEL gene. Therefore, these results should be interpreted with caution regarding the specific agents involved until additional data are available. Anaplasma bovis and related pathogens are widely distributed worldwide and can be found in association with different species of ticks including Amblyomma, and can be detected in different species of wild animals.59–61 In our research, we identified an A. bovis-like isolate in an Amblyomma tick collected from a tortoise. One possible explanation is that free-ranging tortoises scavenge for food around homesteads and interact with humans and livestock, and therefore may acquire ticks that would typically infest peri-domestic animals.62 Furthermore, the water monitor is a common reptile often encountered in city areas in Asian countries, and kabaragoya (the Sri Lankan water monitor) is the most common scavenger animal in the country and may share habitats with tortoises. The Ehrlichia sp. sequence clustering with E. ruminantium was detected in an Amblyomma tick from a pangolin. Ehrlichia ruminantium is the etiological agent of heartwater, a devastating illness affecting livestock in Africa and West Indies. Ehrlichia Panola Mountain agent found in Amblyomma ticks across the United States is closely related to E. ruminantium; however, it causes only mild clinical pathologies and pyrexia in experimentally infected goats.63 A new genotype of Ehrlichia related but distant from E. ruminantium was detected in jaguars and Amblyomma ticks from the Pantanal wetland of Brazil and from crab-eating foxes in southeastern Brazil.64,65 However, their identification was based on limited analysis of the 401-nt dsb gene fragment, which exhibits only 80.7% to 82.5% sequence similarity with the nearest known species, E. ruminantium.64,65
Last, the Ehrlichia sp. genotype detected in R. annulatus represents only a distant sister lineage to E. ewingii. Ehrlichia ewingii is a known human and canine pathogen transmitted by Amblyomma americanum in the United States; however, there is one report describing dsb-gene PCR detection and sequencing of E. ewingii in dogs from Cameroon,66 thus suggesting a broader distribution of this or related genotypes of Ehrlichia. Several other closely related Ehrlichia have been identified in other studies reported from Africa and Asia, so this Ehrlichia lineage may be distributed very broadly. Ehrlichia canis and Anaplasma platys have been reported in different parts of India and other southeastern Asian countries,67–70 but their presence in Sri Lanka is unknown. Further work is required to perform more in-depth analyses of these yet-uncultured Ehrlichia genotypes, and, most importantly, to determine their potential to cause human and veterinary diseases.
In conclusion, although this study demonstrated the presence of several SFG Rickettsia and Anaplasmataceae in ticks from Sri Lanka, some of which may also infect humans and peri-domestic animals, it is very likely that other agents of unknown pathogenicity are also present. National surveillance efforts must take into consideration this diversity of rickettsial agents, because they may exhibit different susceptibilities to therapeutic antibiotics such as rifampin resistance in R. massiliae.33,71 Diagnosis based solely on cross-reactivity with R. conorii antigen is inadequate to resolve the specific rickettsial etiology of human disease because of its poor specificity.15 Efforts to establish a well-characterized collection of regional isolates of Rickettsia and Anaplasmataceae are also important to improve the clinical diagnosis and surveillance of these diseases in Sri Lanka and southern India.
ACKNOWLEDGMENTS
We thank the Office of Global Health, CDC, for the supplemental project funds that supported travel between Sri Lanka and United States, training of R. P. V. J. Rajapakse and R. Premaratna at the CDC, and the fieldwork of G. Dasch conducted in Sri Lanka. We also thank Vijitha Perera of the Wildlife Office of Wasgamuwa National Park for his assistance.
REFERENCES
- 1.↑
Liyanaarachchi DR, Jinadasa HRN, Dilrukshi PRMP, Rajapakse RPVJ, 2013. Epidemiological study on ticks in farm animals in selected areas of Sri Lanka. Trop Agr Res 24: 336–346.
- 2.↑
Agampodi S, Wijerathne B, Weerakoon K, 2016. Situation of Sri Lanka, where autochthonous malaria is no longer a problem, and other infections dominate, such as dengue, leptospirosis and rickettsioses. Curr Opin Infect Dis 29: 446–452.
- 3.↑
Karunasena VM et al., 2019. The first introduced malaria case reported from Sri Lanka after elimination: implications for preventing the re-introduction of malaria in recently eliminated countries. Malar J 18: 210.
- 4.↑
Premaratna R, 2011. Rickettsial infections in Sri Lanka: yesterday, today and tomorrow. J Cey Coll Phys 42: 11–15.
- 5.↑
Premaratna R, 2017. Scrub typhus in Sri Lanka: beyond the stethoscope. J Cey Coll Phy 48: 61–65.
- 6.↑
Premaratna R, Ariyaratna N, Attanayake C, Bandara W, Chandrasena N, de Silva HJ, 2014. Rickettsial infection among military personnel deployed in northern Sri Lanka. BMC Infect Dis 14: 3864.
- 7.↑
Weerakoon KG, Kularatne SAM, Rajapakse J, Adikari S, Udayawarna K, 2017. Revisiting clinico-epidemiological pattern of human rickettsial infections in the central region of Sri Lanka: a hospital based descriptive study. BMC Res Notes 10: 400.
- 8.↑
Kularatne SA, Rajapakse RP, Wickramasinghe WM, Nanayakkara DM, Budagoda SS, Weerakoon KG, Edirisingh JS, Premaratna R, 2013. Rickettsioses in the central hills of Sri Lanka: serological evidence of increasing burden of spotted fever group. Int J Infect Dis 17: e988–e992.
- 9.↑
Kularatne SA, Edirisingha JS, Gawarammana IB, Urakami H, Chenchittikul M, Kaiho I, 2003. Emerging rickettsial infections in Sri Lanka: the pattern in the hilly Central Province. Trop Med Int Health 8: 803–811.
- 10.↑
Reller ME, Bodinayake C, Nagahawatte A, Devasiri V, Kodikara-Arachichi W, Strouse JJ, Flom JE, Ostbye T, Woods CW, Dumler JS, 2012. Unsuspected rickettsioses among patients with acute febrile illness, Sri Lanka, 2007. Emerg Infect Dis 18: 825–829.
- 11.↑
Dalugama C, Gawarammana IB, 2018. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Reports 12: 145.
- 12.↑
Dissanayake NL, Madegedara D, 2011. An unusual case of fatal pulmonary hemorrhage in pregnancy. Lung India 28: 205–208.
- 13.↑
Herath H, Jayasundara J, Senadhira SDN, Kularatne SAM, Kularatne WKS, 2018. Spotted fever rickettsioses causing myocarditis and ARDS: a case from Sri Lanka. BMC Infect Dis 18: 705.
- 14.↑
Kariyawasam AGTA, Palangasinghe DR, Fonseka CL, De Silva PUT, Kanakkahewa TE, Dahanayaka NJ, 2019. Bilateral sensorineural deafness in a young pregnant female presenting with a fever: a rare complication of a reemerging disease-spotted fever group rickettsioses. Case Rep Infect Dis 2019: 5923146.
- 15.↑
Abdad MY, Abou Abdallah R, Fournier PE, Stenos J, Vasoo S, 2018. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol 56: e01728–e17.
- 16.↑
Nagalingam K, Rolain JM, Thevanesam V, Lakkumar F, Gunawardana G, Raoult D, 2009. Spotted fever rickettsioses in children in Sri Lanka. Clin Microbiol Infect 15 (Suppl 2): 330–331.
- 17.↑
Luke N, Munasinghe H, Balasooriya L, Premaratna R, 2017. Widespread subcutaneous necrosis in spotted fever group rickettsioses from the coastal belt of Sri Lanka: a case report. BMC Infect Dis 17: 278.
- 18.↑
Weerakoon K, Kularatne SA, Rajapakse J, Adikari S, Waduge R, 2014. Cutaneous manifestations of spotted fever rickettsial infections in the Central Province of Sri Lanka: a descriptive study. PLoS Negl Trop Dis 8: e3179.
- 19.↑
Cordier C, Tattevin P, Leyer C, Cailleaux M, Raoult D, Angelakis E, 2017. Rickettsia sibirica mongolitimonae infection, Sri Lanka. J Infect Dev Countries 11: 668–671.
- 20.↑
Anderson BE, 1990. The 17-kilodalton protein antigens of spotted fever and typhus group rickettsiae. Ann N Y Acad Sci 590: 326–333.
- 21.↑
Anderson BE, Tzianabos T, 1989. Comparative sequence analysis of a genus-common rickettsial antigen gene. J Bacteriol 171: 5199–5201.
- 22.↑
Kularatne SAM, Fernando R, Selvaratnam S, Narampanawa C, Weerakoon K, Wickramasinghe S, Pathirage M, Weerasinghe V, Bandara A, Rajapakse J, 2018. Intra-aural tick bite causing unilateral facial nerve palsy in 29 cases over 16 years in Kandy, Sri Lanka: is rickettsial aetiology possible? BMC Infect Dis 18: 418.
- 23.↑
Dilrukshi PR, Yasawardene AD, Amerasinghe PH, Amerasinghe FP, 2004. Human otoacariasis: a retrospective study from an area of Sri Lanka. Trans R Soc Trop Med Hyg 98: 489–495.
- 24.↑
Liyanaarachchi DR, Rajakaruna RS, Dikkumbura AW, Rajapakse RP, 2015. Ticks infesting wild and domestic animals and humans of Sri Lanka with new host records. Acta Trop 142: 64–70.
- 25.↑
Liyanaarachchi DR, Rajakaruna RS, Dikkumbura AW, De Silva A, Rajapakse RP, 2015. Ticks (Acari: Ixodidae) infesting five reptile species in Sri Lanka with sixteen new host records. Zootaxa 3964: 146–148.
- 26.↑
Zhyldyz A, Sivakumar T, Igarashi I, Gunasekara E, Kothalawala H, Silva SSP, Yokoyma N, 2019. Epidemiological survey of Anaplasma marginale in cattle and buffalo in Sri Lanka. J Vet Med Sci 81: 1601–1605.
- 28.↑
Moriarity JR, Loftis AD, Dasch GA, 2005. High-throughput molecular testing of ticks using a liquid-handling robot. J Med Entomol 42: 1063–1067.
- 29.↑
Beati L, Keirans JE, 2001. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol 87: 32–48.
- 30.↑
Eremeeva ME et al., 2011. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J Med Entomol 48: 418–421.
- 31.↑
Beati L et al., 2013. Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae), the Cayenne tick: phylogeography and evidence for allopatric speciation. BMC Evol Biol 13: 267.
- 32.↑
Eremeeva ME, Dasch GA, Silverman DJ, 2003. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J Clin Microbiol 41: 5466–5672.
- 33.↑
Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA, 2006. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol 72: 5569–5577.
- 34.↑
Eremeeva ME, Oliveira A, Robinson JB, Ribakova N, Tokarevich NK, Dasch GA, 2006. Prevalence of bacterial agents in Ixodes persulcatus ticks from the Vologda Province of Russia. Ann N Y Acad Sci 1078: 291–298.
- 35.↑
Li JS, Chu F, Reilly A, Winslow GM, 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J Immunol 169: 1419–1425.
- 36.↑
Takano A, Ando S, Kishimoto T, Fujita H, Kadosaka T, Nitta Y, Kawabata H, Watanabe H, 2009. Presence of a novel Ehrlichia sp. in Ixodes granulatus found in Okinawa, Japan. Microbiol Immunol 53: 101–106.
- 37.↑
Weisburg WG, Barns SM, Pelletier DA, Lane DJ, 1991. 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol 173: 697–703.
- 38.↑
Kumar S, Stecher G, Li M, Knyaz C, Tamura K, 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549.
- 39.↑
Newcombe RG, 1998. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17: 2635–2650.
- 40.↑
Andoh M, Sakata A, Takano A, Kawabata H, Fujita H, Une Y, Goka K, Kishimoto T, Ando S, 2015. Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. PLoS One 10: e0133700.
- 41.↑
Eremeeva ME, Stromdahl EY, 2011. Short report: new spotted fever group Rickettsia in a Rhipicephalus turanicus tick removed from a child in eastern Sicily, Italy. Am J Trop Med Hyg 84: 99–101.
- 42.↑
Prakash JA, Sohan Lal T, Rosemol V, Verghese VP, Pulimood SA, Reller M, Dumler JS, 2012. Molecular detection and analysis of spotted fever group Rickettsia in patients with fever and rash at a tertiary care centre in Tamil Nadu, India. Pathog Glob Health 106: 40–45.
- 43.↑
Rolain JM, Mathai E, Lepidi H, Somashekar HR, Mathew LG, Prakash JA, Raoult D, 2006. “Candidatus Rickettsia kellyi,” India. Emerg Infect Dis 12: 483–485.
- 44.↑
Takajo I et al., 2016. Possible case of novel spotted fever group rickettsiosis in traveler returning to Japan from India. Emerg Infect Dis 22: 1079–1082.
- 45.↑
Parola P et al., 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26: 657–702.
- 46.↑
Biswal M, Zaman K, Suri V, Gopi S, Kumar A, Gopi T, Vig S, Sharma N, Bhalla A, 2020. Molecular confirmation and characterization of Rickettsia conorii in north India: a report of three cases. Indian J Med Res 151: 59–64.
- 47.↑
Stokes PH, Walters BJ, 2009. Spotted fever rickettsiosis infection in a traveler from Sri Lanka. J Travel Med 16: 436–438.
- 48.↑
Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE, 2010. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 26: 205–212.
- 49.↑
Nanayakkara DM, Rajapakse RP, Wickramasinghe S, Kularatne SA, 2013. Serological evidence for exposure of dogs to Rickettsia conorii, Rickettsia typhi, and Orientia tsutsugamushi in Sri Lanka. Vector Borne Zoonotic Dis 13: 545–549.
- 50.↑
Garcia-Garcia JC, Portillo A, Nunez MJ, Santibanez S, Castro B, Oteo JA, 2010. A patient from Argentina infected with Rickettsia massiliae. Am J Trop Med Hyg 82: 691–692.
- 51.↑
Parola P, Socolovschi C, Jeanjean L, Bitam I, Fournier PE, Sotto A, Labauge P, Raoult D, 2008. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl Trop Dis 2: e338.
- 52.↑
Chochlakis D, Ioannou I, Sandalakis V, Dimitriou T, Kassinis N, Papadopoulos B, Tselentis Y, Psaroulaki A, 2012. Spotted fever group rickettsiae in ticks in Cyprus. Microb Ecol 63: 314–323.
- 53.↑
Kimita G, Mutai B, Nyanjom SG, Wamunyokoli F, Waitumbi J, 2016. Phylogenetic variants of Rickettsia africae, and incidental identification of “Candidatus Rickettsia moyalensis” in Kenya. PLoS Negl Trop Dis 10: e0004788.
- 54.↑
Dietrich M et al., 2014. Rickettsia spp. in seabird ticks from western Indian Ocean islands, 2011–2012. Emerg Infect Dis 20: 838–842.
- 55.↑
Waner T, Keysary A, Eremeeva ME, Din AB, Mumcuoglu KY, King R, Atiya-Nasagi Y, 2014. Rickettsia africae and Candidatus Rickettsia barbariae in ticks in Israel. Am J Trop Med Hyg 90: 920–922.
- 56.↑
Robertson RG, Wiseman CL Jr , Traub R, 1970. Tick-borne rickettsiae of the spotted fever group in West Pakistan: I. Isolation of strains from ticks in different habitats. Am J Epidemiol 92: 382–394.
- 57.↑
Robertson RG, Wisseman CL Jr , 1973. Tick-borne rickettsiae of the spotted fever group in West Pakistan: II. Serological classification of isolates from West Pakistan and Thailand: evidence for two new species. Am J Epidemiol 97: 55–64.
- 58.↑
Hocquart M, Drouet H, Levet P, Raoult D, Parola P, Eldin C, 2019. Cellulitis of the face associated with SENLAT caused by Rickettsia slovaca detected by qPCR on scalp eschar swab sample: an unusual case report and review of literature. Ticks Tick Borne Dis 10: 1142–1145.
- 59.↑
Andre MR, 2018. Diversity of Anaplasma and Ehrlichia/Neoehrlichia agents in terrestrial wild carnivores worldwide: implications for human and domestic animal health and wildlife conservation. Front Vet Sci 5: 293.
- 60.↑
Gofton AW, Waudby HP, Petit S, Greay TL, Ryan UM, Irwin PJ, 2017. Detection and phylogenetic characterisation of novel Anaplasma and Ehrlichia species in Amblyomma triguttatum subsp. from four allopatric populations in Australia. Ticks Tick Borne Dis 8: 749–756.
- 61.↑
Koh FX, Kho KL, Panchadcharam C, Sitam FT, Tay ST, 2016. Molecular detection of Anaplasma spp. in pangolins (Manis javanica) and wild boars (Sus scrofa) in Peninsular Malaysia. Vet Parasitol 227: 73–76.
- 62.↑
Omondi D, Masiga DK, Fielding BC, Kariuki E, Ajamma YU, Mwamuye MM, Ouso DO, Villinger J, 2017. Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent islands of Lake Victoria and Lake Baringo, Kenya. Front Vet Sci 4: 73.
- 63.↑
Yabsley MJ, Loftis AD, Little SE, 2008. Natural and experimental infection of white-tailed deer (Odocoileus virginianus) from the United States with an Ehrlichia sp. closely related to Ehrlichia ruminantium. J Wildl Dis 44: 381–387.
- 64.↑
Widmer CE, Azevedo FC, Almeida AP, Ferreira F, Labruna MB, 2011. Tick-borne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis 11: 1001–1005.
- 65.↑
Almeida AP, Souza TD, Marcili A, Labruna MB, 2013. Novel Ehrlichia and Hepatozoon agents infecting the crab-eating fox (Cerdocyon thous) in southeastern Brazil. J Med Entomol 50: 640–646.
- 66.↑
Ndip LM, Ndip RN, Esemu SN, Dickmu VL, Fokam EB, Walker DH, McBride JW, 2005. Ehrlichial infection in Cameroonian canines by Ehrlichia canis and Ehrlichia ewingii. Vet Microbiol 111: 59–66.
- 67.↑
Mittal M, Kundu K, Chakravarti S, Mohapatra JK, Nehra K, Sinha VK, Sanjeeth BS, Churamani CP, Kumar A, 2017. Canine monocytic ehrlichiosis among working dogs of organised kennels in India: a comprehensive analyses of clinico-pathology, serological and molecular epidemiological approach. Prev Vet Med 147: 26–33.
- 68.↑
Malik MI, Qamar M, Ain Q, Hussain MF, Dahmani M, Ayaz M, Mahmood AK, Davoust B, Shaikh RS, Iqbal F, 2018. Molecular detection of Ehrlichia canis in dogs from three districts in Punjab (Pakistan). Vet Med Sci 4: 126–132.
- 69.↑
Sarma K, Nachum-Biala Y, Kumar M, Baneth G, 2019. Molecular investigation of vector-borne parasitic infections in dogs in Northeast India. Parasit Vectors 12: 122.
- 70.↑
Hmoon MM, Htun LL, Thu MJ, Chel HM, Thaw YN, Win SY, Chan Soe N, Khaing Y, Thein SS, Bawm S, 2021. Molecular prevalence and identification of Ehrlichia canis and Anaplasma platys from dogs in Nay Pyi Taw Area, Myanmar. Vet Med Int 2021: 8827206.
- 71.↑
Rolain JM, Maurin M, Vestris G, Raoult D, 1998. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother 42: 1537–1541.
- 72.↑
Roux V, Raoult D, 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol 50: 1449–1455.
- 73.↑
Sekeyova Z, Roux V, Raoult D, 2001. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D,’ which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol 51: 1353–1360.