INTRODUCTION
Secondary hemophagocytic lymphohistiocytosis (HLH) in association with typhoid fever is known since early 1970s.1 Clinical reports of invasive non-typhoidal Salmonella (iNTS) infections triggering HLH are scarce. Salmonella Typhimurium has been shown to trigger HLH in murine and in vitro models.2 In either of the scenarios, in vivo or in vitro, Salmonella Enteritidis has not been reported to be associated with HLH.
Second, ceftriaxone resistance, although not uncommon in iNTS infections, is usually more commonly encountered with S. Typhimurium than S. Enteritidis.3 Herein, we report an immunocompetent healthy male child with ceftriaxone-resistant invasive S. Enteritidis infection complicated by secondary HLH who was successfully managed with intravenous (IV) meropenem.
CASE REPORT
A 12-year-old male child presented with a 7-day history of fever, intermittent headache, and decreased appetite. Examination showed a well-nourished (height: 140 cm, weight: 35 kg, and body mass index: 17.9 kg/m2), sick-looking febrile child (102°F) with coated tongue, hepatomegaly (liver palpable 5 cm below the right costal margin with a span of 12 cm), and a palpable spleen tip. Rest of the physical examination was unremarkable. Investigations (value [normal range]) carried out at admission showed a hemoglobin level of 116 g/L that decreased to 88 g/L (11.5–15.5) over 1 week, leukopenia (3.60 × 109/L [5.0–14.5], polymorphs 68%, lymphocytes 27%, and monocytes 5%), thrombocytopenia (85 × 109/L [150–400]), an elevated procalcitonin level of 47.4 ng/mL (< 0.5), and an elevated C-reactive protein (CRP) level of 234.77 mg/L (< 6). He also had elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of 70 (< 45) and 161 (< 45) U/L, respectively. Reversal of albumin (2.8 g/dL [3.5–5.6])-to-globulin (3.4 g/dL) ratio (0.82 [1.2–1.8]) and elevated urea (64 [15–40] mg/dL) and creatinine (1.05 [0.31–0.88] mg/dL) levels were also noted. Initial investigations for infectious etiology including malarial parasite smear; serology for hepatitis A, B, C, and E viruses, dengue virus non-structural protein 1 antigen and IgM antibodies, Epstein Barr virus viral capsid antigen IgM VCA, Brucella serology; urine routine and culture; cerebrospinal fluid cytology; and biochemistry were noncontributory, except for positive Widal agglutination tests (TH-1:320 and TO-1:160 [< 1:80], on two occasions).
Enteric fever was considered, and he was empirically started on IV ceftriaxone at 100 mg/kg/day. However, he had no improvement in clinical condition even after 4 days of IV ceftriaxone. Over the hospital stay, he also developed tachypnea and worsening sensorium. Laboratory investigations revealed progressive elevation in inflammatory parameters (CRP: 272.1 [< 6] mg/L and procalcitonin: 84.1 [< 0.5] ng/mL) and transaminases (AST: 279 [< 45], ALT: 109 [< 45], alkaline phosphatase: 583 [200–495] U/L, and gamma-glutamyl transferase: 955 [5–24] U/L).
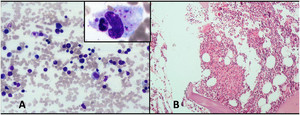
Other differentials such as tuberculosis, fungal infections, malignancy, and HLH were considered, and the child was investigated further, including bone marrow examination and cultures. Subsequent workup (on day 4 of hospital stay) showed an erythrocyte sedimentation rate of 7 mm in the first hour (0–20), a ferritin level of 1,316 (10–300) ng/mL, a fibrinogen level of 1.1 (1.8–3.6) g/L, and a triglyceride level of 171 (< 100) mg/dL (Supplemental Table 1). Meanwhile, blood and, subsequently, bone marrow cultures showed growth of non-lactose fermenting Gram-negative bacilli. The isolate was identified by matrix-assisted laser ionization time-of-flight, mass spectrometry analysis (Microflex LT Biotyper Instrument Bruker Daltonics, Bremen, Germany) (score 2.0) as Salmonella species and was further confirmed to be S. Enteritidis by serotyping (obtained from the National Salmonella Centre, Central Research Institute [CRI], Kasauli, India). The sensitivity of S. Enteritidis was performed by using the disc diffusion method in accordance with the Central Laboratory Standards Institute (CLSI 2020). The isolate was resistant to ceftriaxone, cefixime, trimethoprim–sulfamethoxazole, aminoglycosides with intermediate susceptibility to piperacillin/tazobactam, cefoperazone/sulbactam, and cefepime. The isolate was susceptible to fluoroquinolones and carbapenems. Bone marrow examination revealed hemophagocytosis, granulomas, and necrotic debris without any evidence of malignancy (Figure 1). The bone marrow was hypocellular for age, with approximately 50–60% cellularity. The histiocytes showing hemophagocytosis were frequent, with approximately 1 histiocyte/10 high-power field (×1,000 magnification). Workup for tuberculosis including tuberculin testing and gastric lavage for acid-fast bacilli on two occasions was negative. Chest radiograph was normal. Ultrasonography of the abdomen revealed hepatosplenomegaly with no evidence of intra-abdominal collections or enlarged lymph nodes.



(A) Bone marrow aspirate showing normal marrow cells with an increase in histiocytes and hemophagocytosis (highlighted in inset) (Giemsa and May Grunwald Giemsa stain ×200 and inset ×1,000). (B) Bone marrow biopsy showing the presence of granuloma along with necrosis (hematoxylin and eosin stain ×200).
Citation: The American Journal of Tropical Medicine and Hygiene 103, 6; 10.4269/ajtmh.20-0566
The patient was started on IV meropenem (120 mg/kg/day in three divided doses) along with IV dexamethasone (10 mg/m2/day initially, converted to oral, and subsequently tapered over 2 weeks) in view of secondary HLH. The patient responded to the therapy and became afebrile within 2 days. Also, the clinical condition and laboratory parameters improved gradually. Blood culture repeated after 1 week of IV meropenem was sterile. He was given 2 weeks of therapy with IV meropenem. Bone marrow fungal culture came sterile after 15 days on incubation.
Considering the possibility of an underlying susceptibility to develop invasive iNTS or HLH, he was investigated further. His serology for HIV was nonreactive. Preliminary immunology workup showed normal lymphocyte subsets, normal immunoglobulin levels, normal dihydrorhodamine 123 assay, normal expression of interferon γ receptor subunit 1 on white blood cell subsets, and normal perforin expression on natural killer cells. Hemoglobin electrophoresis was normal (hemoglobin A: 95%, hemoglobin A 2: 2.6%, and hemoglobin F: 1.7%). He was clinically doing well, had no evidence of organomegaly, and blood counts were normal at the third month follow-up.
DISCUSSION
Our case highlights two important findings: secondary HLH in the context of invasive ceftriaxone-resistant S. Enteritidis infection and its successful treatment with IV meropenem. Invasive iNTS is an emerging public health concern. In contrast to iNTS infections, the global incidence of typhoid and paratyphoid fever is decreasing gradually over the last three decades. A global estimate for the year 2017 suggested enteric fever to have caused 27 times more cases than iNTS infections; however, iNTS infections contributed ≈ 36% to the combined mortality. Case fatality of ≈ 9% for iNTS infections in children was much higher than that of typhoid and paratyphoid fever (≈ 1%).4,5 Increased risk of drug resistance and secondary HLH may be some of the important factors resulting in a higher case fatality rate in iNTS infections than enteric fever.
Recent reviews suggest drug resistance to be commoner in iNTS infections than S. Typhi/Paratyphi infections.6,7 Monotherapy with meropenem has been used for treatment of extensively drug-resistant S. Typhi infections in children with good results.8 However, the literature describing meropenem monotherapy for ceftriaxone-resistant iNTS infections is scarce.9 Our case was successfully treated with 2 weeks of IV meropenem monotherapy that resulted in prompt defervescence of fever. In our case, bone marrow biopsy also showed granuloma with necrotic areas. This finding has been reported previously in patients with enteric fever10; however, such reports are limited in iNTS infections.
Many tropical infections such as malaria, scrub typhus, dengue, and leishmaniasis have been reported to trigger HLH (Supplemental Table 2). Besides, there are several reports of Salmonella sp. infection with HLH; however, reports of HLH with iNTS are only handful Supplemental Table 3). Secondary HLH has not been reported previously with S. Enteritidis in immunocompetent children. An old French language report and a recent Chinese report describe HLH in association with invasive S. Typhimurium infection in children with chronic granulomatous disease (CGD).11,12 The real trigger, in this case, may be debatable, as CGD, in itself, is known to get complicated with HLH.12,13 Our child did not have any clinical or laboratory evidence suggestive of inborn error of immunity. A recent Turkish report also describes invasive S. Typhimurium infection in an apparently immunocompetent child.14 Secondary HLH has been described in association with invasive S. choleraesuis infections as well.15
Most of the cases of invasive salmonellosis have been diagnosed to have HLH on the basis of the HLH-2004 criteria. Our patient fulfilled both the HLH-2004 and HScore16 criteria for diagnosis of HLH (HScore of 249, probability of having reactive hemophagocytic syndrome: 99.4%; Supplemental Table 1). However, literature review suggests that assessment of soluble interleukin-2 receptor levels or natural killer cell cytotoxicity has been performed in a very small number of patients with infection triggered HLH (Supplemental Tables 2 and 3). We were not able to perform these tests in our patient. Secondary HLH (fulfilling diagnostic criteria) may only represent the “tip of the iceberg,” with multi-organ dysfunction, systemic inflammatory response syndrome, and hyperferritinemia being other potential unrecognized manifestations of the same pathogenetic mechanism.17 Multicentric studies are needed to determine the actual risk of HLH in iNTS infections vis-à-vis enteric fever. Irrespective of all microbial characteristics, appropriate antimicrobials are the mainstay of therapy in case of infection and secondary HLH. This principle holds true not only for salmonellosis but also for most of the infections triggering HLH. Immunomodulatory/suppressive therapy is usually required in severe cases or with poor response to antimicrobial therapy (Supplemental Tables 2 and 3). Steroids were given in addition to antimicrobial therapy in our case because of the worsening clinical condition.
Taken together, all these findings suggest clinicians to give special consideration to iNTS infections and its complications, especially in settings endemic for enteric fever. This can go a long way in reducing mortality and morbidity in children with iNTS infections, as specific vaccines for protection against iNTS infections significantly lag behind the already well-tested and approved typhoid vaccines.18
ACKNOWLEDGMENT
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 2.↑
Pilonieta MC, Moreland SM, English CN, Detweiler CS, 2014. Salmonella enterica infection stimulates macrophages to hemophagocytose. mBio 5: e02211.
- 3.↑
Kariuki S, Onsare RS, 2015. Epidemiology and genomics of invasive nontyphoidal Salmonella infections in Kenya. Clin Infect Dis 61 (Suppl 4): S317–S324.
- 4.↑
GBD 2017 Non-Typhoidal Salmonella Invasive Disease Collaborators, 2019. The global burden of non-typhoidal Salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19: 1312–1324.
- 5.↑
GBD 2017 Typhoid and Paratyphoid Collaborators, 2019. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19: 369–381.
- 6.↑
Lester R, Musicha P, van Ginneken N, Dramowski A, Hamer DH, Garner P, Feasey NA, 2020. Prevalence and outcome of bloodstream infections due to third-generation cephalosporin-resistant Enterobacteriaceae in sub-Saharan Africa: a systematic review. J Antimicrob Chemother 75: 492–507.
- 7.↑
Browne AJ et al. 2020. Drug-resistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis. BMC Med 18: 1.
- 8.↑
Hussain A, Satti L, Hanif F, Zehra NM, Nadeem S, Bangash TM, Peter A, 2019. Typhoidal Salmonella strains in Pakistan: an impending threat of extensively drug-resistant Salmonella Typhi. Eur J Clin Microbiol Infect Dis 38: 2145–2149.
- 9.↑
Altinel Açoğlu E, Sari E, Şahin G, Oğuz MM, Akçaboy M, Zorlu P, Senel S, 2019. Kikuchi-Fujimoto disease triggered by Salmonella Enteritidis in a child with concurrent auto-immune thyroiditis and papilloedema. Paediatr Int Child Health 38: 298–301.
- 10.↑
Lee WS, Kim JH, Choi TY, 2004. Bone marrow granulomas in Salmonella Paratyphi A infection. Br J Haematol 127: 242.
- 11.↑
Benz-Lemoine E, Bordigoni P, Schaack JC, Briquel E, Chiclet AM, Olive D, 1983. Histiocytose réactionnelle systémique avec hémophagocytose et troubles de l’hémostase associés à une granulomatose septique [systemic reactive histiocytosis with hemophagocytosis and hemostasis disorders associated with septic granulomatosis] [article in French]. Arch Fr Pediatr 40: 179–182.
- 12.↑
Wei A, Ma H, Zhang L, Li Z, Zhang Q, Wang D, Zhang L, Lian H, Zhang R, Wang T, 2020. Hemophagocytic lymphohistiocytosis resulting from a cytokine storm triggered by septicemia in a child with chronic granuloma disease: a case report and literature review. BMC Pediatr 20: 100.
- 13.↑
Parekh C, Hofstra T, Church JA, Coates TD, 2011. Hemophagocytic lymphohistiocytosis in children with chronic granulomatous disease. Pediatr Blood Cancer 56: 460–462.
- 14.↑
Yaşar Durmuş S, Tanır G, Öz FN, Aydın Teke T, Kaman A, 2020. Salmonella ser. Typhimurium bacteremia related hemophagocytic lymphohistiocytosis: a case report. J Pediatr Inf 14: e35–e37.
- 15.↑
Song M, Qiu H, 2017. Clinical analysis of Gram-negative bacillisepticemia-associated hemophagocytic lymphohistiocytosis. Blood 130 (Suppl 1): 2292.
- 16.↑
Fardet L et al. 2014. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 66: 2613–2620.
- 17.↑
Crayne C, Cron RQ, 2019. Pediatric macrophage activation syndrome, recognizing the tip of the iceberg. Eur J Rheumatol 7 (Suppl 1): 1–8.
- 18.↑
Kariuki S, Mbae C, Onsare R, Kavai SM, Wairimu C, Ngetich R, Ali M, Clemens J, Dougan G, 2019. Multidrug-resistant nontyphoidal Salmonella hotspots as targets for vaccine use in management of infections in endemic settings. Clin Infect Dis 68 (Suppl 1): S10–S15.