NOT JUST DISEASE X
In the 21st century, there have been several major outbreaks of novel pathogens, such as SARS-CoV-1, Middle Eastern respiratory virus, severe fever with thrombocytopenia syndrome virus, and SARS-CoV-2.1–4 Novel pathogens continue to pose a significant threat, especially as the global community continues to be more interconnected and as human activities drive us and animal populations to closer interaction, increasing the risk for spillover events.5,6 In 2018, the WHO codified this concept of an unknown threat when it included “Disease X” on its list of priority pathogens.
The WHO formally defined Disease X as representing “the knowledge that a serious international epidemic could be caused by a pathogen currently unknown to cause human disease.”7 This unprecedented move acknowledged that the pathogenome of the world still held many unknowns, and encouraged initiatives aimed at enhanced surveillance and preparedness. By including Disease X on the priority pathogen list, the WHO signaled that research and development should account for the unknown threat. As SARS-CoV-2 and previously emergent novel pathogens have shown us, the novel pathogen poses more than just a theoretical threat. The pandemic has brought increased references to Disease X in preparing the global community for subsequent threats. But calls to action for pandemic preparedness under the banner of Disease X are too narrowly focused, and a balance must be struck between accounting for unknown dangers and maintaining focus on already identified, often neglected infectious diseases of public health importance.
In fact, some of the most significant outbreaks in the past 20 years have been from previously identified pathogens, such as cholera in Zimbabwe 2008; pandemic swine flu in 2009; chikungunya and Zika viruses in South and Central America 2014 and 2015, respectively; Ebola virus in West Africa 2014–2015; and yellow fever virus in Brazil 2016.8–13 In addition, during the writing of this piece, there has been identification of monkeypox virus in the Europe and elsewhere and detection of polio virus in Mozambique after 30 years, detection in the UK after 40 years as well as the United States (New York).14–17 Introductions, expansions, and reemergence events highlight that discreet characterization of known pathogens is also not sufficient. Some pathogens seem to be adequately characterized, but what we know may in fact become insufficient given significant changes to the ecology of the pathogen. For example, changes due to urbanization and deforestation encourage spillovers and introductions into new geographies; climate change leads to expansion of vectors and their associated pathogens; and social issues (e.g., the rise in antivaccination movements or forced migration) alter the demographics of human communities.18 All these factors essentially provide more opportunities for pathogen contact with susceptible hosts, affording the means for emergence and the potential for fulminant outbreaks. Thus, although there is an important need for virus hunters to continue to discover the identities of potential Disease X pathogens, prospective and steady basic research and public health preparedness needs a broader framing for efficient and expedient allocation of resources and ultimately translation of results into programmatic development and optimization.
A broader, One Health-aligned framework.
One Health is the understanding that health of humans, animals, and the environment are interconnected and is a critical paradigm for framing future research for optimized planetary health.19 One Health is the context in which specific problems should be set to formulate holistic and balanced solutions. One Health encompasses myriad things currently threatening global health, including (but not limited to) infectious diseases, noncommunicable diseases, environmental disasters, and climate change. Disease X as a focus for surveillance does not intuitively put things into the One Health paradigm because it focuses on the identity of a pathogen and, in some respects, whether it has been previously identified or not.
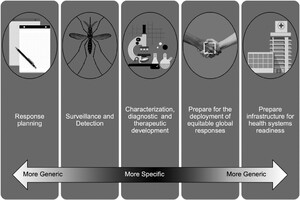
Developing subframeworks that are One Health aligned but that address specific aspects of health focuses efforts, identifies the most relevant stakeholders, and leads to balanced and efficient responses. As an example, we discuss one such subframework to address emerging infectious threats specifically and associated preparedness and response. We propose the framework of Disease
We propose defining Disease
In addition to specific therapeutics development, pandemic research and development should include and prioritize the transmission ecology of pathogens, both natural and anthropogenic. As SARS-CoV-2 has demonstrated, social and political pressures, as well as global equity issues have all contributed to the persistence of the virus, even when therapeutics were deployed in record time.20,21 Deficiencies in understanding of the transmission mode(s) early in the pandemic delayed masking and other nonpharmaceutical intervention recommendations which were further exacerbated by delayed and/or poor science communication.22,23 Further, there are a multitude of viruses for which diagnostic capabilities are currently non-existent or severely lagging, as was highlighted by the West African Ebola virus (2014–2015) outbreak and the Zika virus outbreak in South America (2015–2016).24 Additionally, a lack of quickly available, sensitive, and specific diagnostics may create a reliance on clinical syndrome for diagnosis, which creates an additional issue of possible misdiagnosis.25
Critically, by shifting the novelty of the “next big thing” from Disease X, potential threats from already identified (possibly neglected) pathogens will not be discounted. Additionally, Disease
Finally, Disease



Championing a broad framework for preparedness that is One Health aligned allows for development of flexible programs that are progressively specific, as necessary, but exploits common aspects of pathogen transmission to allow for generic planning programs and response protocols.
Citation: The American Journal of Tropical Medicine and Hygiene 107, 6; 10.4269/ajtmh.22-0341
Although perhaps intuitive, the framework of Disease
REFERENCES
- 1.↑
WHO , 2003. Global surveillance for severe acute respiratory syndrome (SARS). Wkly Epidemiol Rec 78: 100–119. https://apps.who.int/iris/bitstream/handle/10665/232131/WER7814_100-105.PDF.
- 2.↑
Zaki AM , van Boheemen S , Bestebroer TM , Osterhaus AD , Fouchier RA , 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367: 1814–1820.
- 3.↑
Munster VJ , Koopmans M , van Doremalen N , van Riel D , de Wit E , 2020. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med 382: 692–694.
- 4.↑
Yu X-J et al., 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364: 1523–1532.
- 6.↑
Carlson CJ , Albery GF , Phelan A , 2021. Preparing international cooperation on pandemic prevention for the Anthropocene. BMJ Glob Health 6: 3.
- 7.↑
World Health Organization , 2018. Prioritizing Diseases for Research and Development in Emergency Contexts. Available at: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts.
- 8.↑
Mason PR , 2009. Zimbabwe experiences the worst epidemic of cholera in Africa. J Infect Dev Ctries 3: 148–151.
- 9.↑
Peiris JS , Poon LL , Guan Y , 2009. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol 45: 169–173.
- 10.↑
Leparc-Goffart I , Nougairede A , Cassadou S , Prat C , de Lamballerie X , 2014. Chikungunya in the Americas. Lancet 383: 514.
- 11.↑
Baize S et al., 2014. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 371: 1418–1425.
- 12.↑
Zanluca C , Melo VC , Mosimann AL , Santos GI , Santos CN , Luz K , 2015. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 110: 569–572.
- 13.↑
de Oliveira Figueiredo P et al., 2020. Re-emergence of yellow fever in Brazil during 2016–2019: challenges, lessons learned, and perspectives. Viruses 12: 11.
- 14.↑
International Society for Infectious Diseases , 2022. Monkeypox—UK, (03): Local Transmission. ProMed. Available at: https://promedmail.org/promed-post/?id=8703317. Accessed May 18, 2022.
- 15.↑
United Nations , 2022. First Polio Outbreak in 30 Years Declared in Mozambique. Available at: https://news.un.org/en/story/2022/05/1118502. Accessed May 18, 2022.
- 16.↑
Doucleff M , 2022. Polio is Found in the U.K. for the First Time in Nearly 40 Years. Here’s What it Means. Available at: https://www.npr.org/sections/goatsandsoda/2022/06/22/1106711204/polio-found-in-u-k-for-the-first-time-in-nearly-40-years-heres-what-it-means.
- 17.↑
Wade G , 2022. New York battles polio. New Scientist 255: 7. doi: 10.1016/S0262-4079(22)01648-7.
- 18.↑
Gibb R , Franklinos LHV , Redding DW , Jones KE , 2020. Ecosystem perspectives are needed to manage zoonotic risks in a changing climate. BMJ 371: m3389.
- 19.↑
World Health Organization , 2021. Tripartite and UNEP support OHHLEP’s Definition of “One Health.” Geneva, Switzerland: WHO.
- 20.↑
Yu MA , Shen AK , Ryan MJ , Boulanger LL , 2021. Coordinating COVID-19 vaccine deployment through the WHO COVID-19 Partners Platform. Bull World Health Organ 99: 171.
- 22.↑
Cheng KK , Lam TH , Leung CC , 2022. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity. Lancet 399: e39–e40.
- 23.↑
Escandon K et al., 2021. COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection. BMC Infect Dis 21: 710.
- 24.↑
Kelly-Cirino CD et al., 2019. Importance of diagnostics in epidemic and pandemic preparedness. BMJ Glob Health 4 (Suppl 2): e001179.
- 25.↑
Dutuze MF , Ingabire A , Gafarasi I , Uwituze S , Nzayirambaho M , Christofferson RC , 2020. Identification of Bunyamwera and possible other orthobunyavirus infections and disease in cattle during a Rift Valley fever outbreak in Rwanda in 2018. Am J Trop Med Hyg 103: 183–189.
- 26.↑
Robert MA , Christofferson RC , Weber PD , Wearing HJ , 2019. Temperature impacts on dengue emergence in the United States: investigating the role of seasonality and climate change. Epidemics 28: 100344.
- 27.↑
Hotez P , 2019. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 85: 912–914.
- 28.↑
Anoko JN et al., 2020. Community engagement for successful COVID-19 pandemic response: 10 lessons from Ebola outbreak responses in Africa. BMJ Glob Health 4 (Suppl 7). doi: 10.1136/bmjgh-2020-003121.
- 29.↑
Tagliacozzo S , Albrecht F , Ganapati NE , 2021. International perspectives on COVID-19 communication ecologies: public health agencies’ online communication in Italy, Sweden, and the United States. Am Behav Sci 65: 934–955.